فبراير . 17, 2025 13:08 Back to list
Multi-Drug Panel Rapid Test Cup
The realm of pharmaceutical innovation is constantly evolving, presenting unique challenges and unprecedented solutions. One intriguing development is the concept of a cup drug. This term, while not mainstream, signifies a new approach to personalized medicine—a path where pharmaceutical delivery meets convenience and user-friendliness.
Moreover, regulatory pathways for approval would need to evolve alongside this innovation. Current models may not fully accommodate the complexities of a personalized, multi-drug system. Advocates for such technology would work in tandem with governing bodies to establish new guidelines, ensuring safety and efficacy while fostering an environment conducive to innovative breakthroughs. In terms of authoritativeness, the development of such a system would likely involve collaboration between pharmaceutical giants and biotechnology firms. Drawing on decades of research and development knowledge, these collaborations aim to merge cutting-edge delivery technology with traditional pharmaceutical wisdom, accelerating the path toward personalized, patient-friendly medical solutions. Trustworthiness, an essential pillar in the acceptance of any new medical technology, would be built through rigorous clinical testing and transparent results sharing. Real-world evidence from clinical trials would highlight the safety profile and effectiveness of these formulations, building confidence among healthcare practitioners and patients alike. In conclusion, the cup drug concept is a visionary leap in pharmaceutical care. It marries the sophistication of modern genomics with the practical demands of everyday life, emphasizing personalized healthcare. By addressing patient compliance, ease of administration, and therapeutic precision, this approach reflects the future of medicine—a future where patient-centric solutions are not just innovations but essential standards of care.
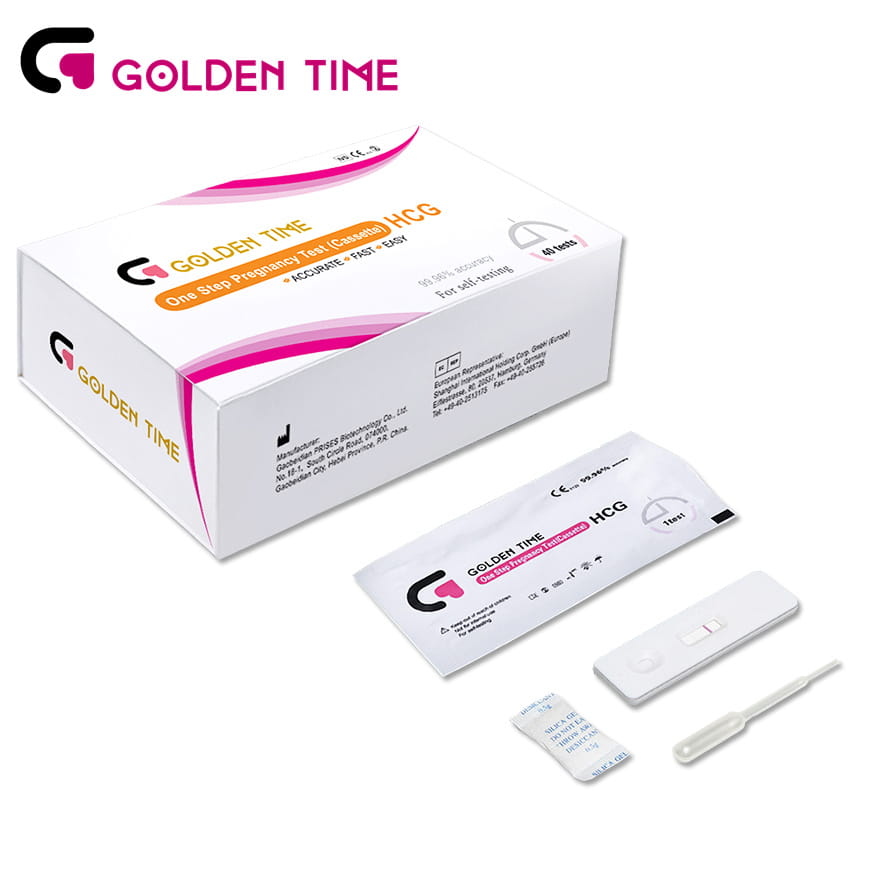
Moreover, regulatory pathways for approval would need to evolve alongside this innovation. Current models may not fully accommodate the complexities of a personalized, multi-drug system. Advocates for such technology would work in tandem with governing bodies to establish new guidelines, ensuring safety and efficacy while fostering an environment conducive to innovative breakthroughs. In terms of authoritativeness, the development of such a system would likely involve collaboration between pharmaceutical giants and biotechnology firms. Drawing on decades of research and development knowledge, these collaborations aim to merge cutting-edge delivery technology with traditional pharmaceutical wisdom, accelerating the path toward personalized, patient-friendly medical solutions. Trustworthiness, an essential pillar in the acceptance of any new medical technology, would be built through rigorous clinical testing and transparent results sharing. Real-world evidence from clinical trials would highlight the safety profile and effectiveness of these formulations, building confidence among healthcare practitioners and patients alike. In conclusion, the cup drug concept is a visionary leap in pharmaceutical care. It marries the sophistication of modern genomics with the practical demands of everyday life, emphasizing personalized healthcare. By addressing patient compliance, ease of administration, and therapeutic precision, this approach reflects the future of medicine—a future where patient-centric solutions are not just innovations but essential standards of care.
Latest news
-
Dengue NS1 Rapid Diagnostic Test Kit
NewsMar.07,2025
-
Dengue NS1 Rapid Diagnostic Test Kit
NewsMar.07,2025
-
Dengue NS1 Rapid Diagnostic Test Kit
NewsMar.07,2025
-
Transferrin Rapid Test Cassette Tumor Marker TF Card
NewsMar.07,2025
-
Malaria Pf Pan Rapid Diagnostic Test Kit
NewsMar.07,2025
-
malaria pf / pan ag rapid test
NewsMar.07,2025

