Oct . 24, 2025 10:40 Back to list
Canine Corona Guide & Support | Fast Facts & Vet-Backed
Field Notes from Clinics: Fast Screening for Canine Corona
I visit a lot of veterinary practices. One recurring theme? Time is everything when a coughing pup or a diarrheic litter walks in. Rapid screening kits have become the unsung heroes behind the desk, and—to be honest—the latest lateral-flow formats are much better than the paper-strip relics we used a decade ago.

What it is and why clinics care
The Canine Coronavirus Antigen Rapid Test Kit is a lateral flow immunochromatographic assay designed for qualitative detection of CCV antigen in dog feces. In everyday terms: a quick, disposable test that helps triage gastroenteric cases. The manufacturer is based in No.136, Shiji West Road, Gaobeidian City, 074000, Hebei Province, P.R. China—worth noting for import paperwork and lead times.
Many customers say they reach for it during intake screening in shelters, pre-boarding checks in kennels, or when an outbreak is suspected alongside parvo. The same supplier also offers configurations covering FPV, FCV, FHV, COV, CPV, CAV, and CCV—handy if you want a unified brand across panels.
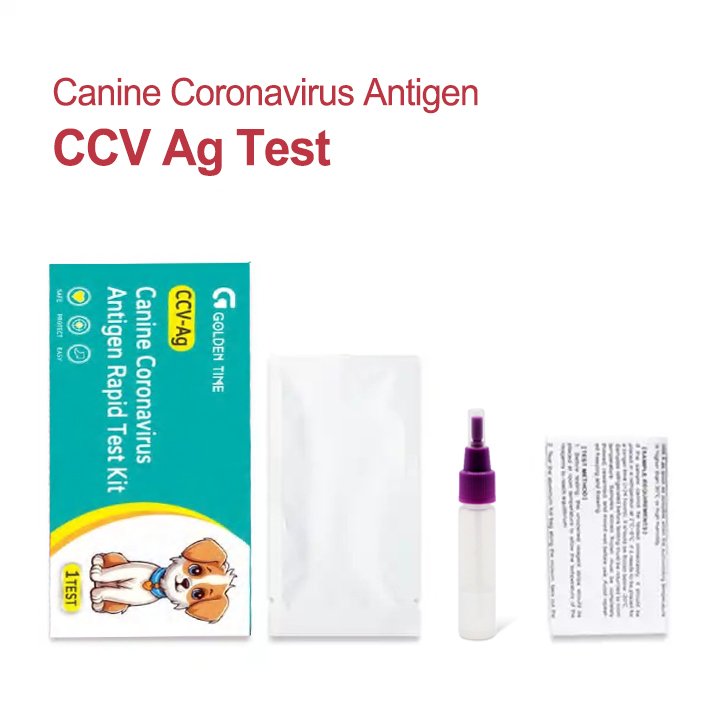
How it works (short version)
-
- Materials: nitrocellulose membrane, colloidal-gold–labeled anti-CCV monoclonal antibodies, sample pad, absorbent pad, plastic cassette.
- Method: lateral flow immunochromatography; visual read at 10–15 minutes.
- Sample: fresh feces (swab or small portion in buffer).
- Internal controls: control line (C) must appear; test line (T) indicates antigen presence.
- Service life: ≈18–24 months at 2–30°C (real-world storage affects this).
- Typical verification: CLSI EP12-style evaluation; accelerated aging at 45°C (6 weeks) often used for stability modeling.
Performance and standards (what labs ask me)
Internal validation (n≈200 clinical fecal samples) reported sensitivity ≈92–95% and specificity ≈97–99% versus a reference PCR. That’s in line with modern lateral-flow expectations; actual performance varies with sample quality and timing of shedding. Labs typically benchmark against CLSI EP12-A2 for qualitative tests and follow ISO 13485 quality systems at the factory. Some buyers also request CE/UKCA veterinary declarations; check your market’s rules.

Use cases I see most
-
- Shelter intake triage during diarrhea clusters.
- Kennel pre-boarding screening when vaccination history is patchy.
- Mobile vets needing fast isolation decisions.
- General practices co-testing with parvo where co-infections are suspected.
Product specifications
| Format | Single-use cassette, lateral flow |
| Target | Canine coronavirus antigen (CCV Ag) |
| Sample | Feces (swab in buffer) |
| Time to result | ≈10–15 min |
| Storage | 2–30°C, dry; do not freeze |
| Shelf life | 18–24 months (unopened) |
| Certs (typ.) | ISO 13485 QMS; regional registrations vary |

Vendor snapshot (market reality)
| Vendor | Certs | MOQ | Lead Time | Customization | Price (≈) |
|---|---|---|---|---|---|
| PrisesBio (China) | ISO 13485; vet CE claims (market-dependent) | 1–500 kits | 7–15 days | Private label, multi-language IFU | $•–$$ |
| Vendor A | ISO 13485 | 1,000+ | 3–5 weeks | Box art & barcode only | $$ |
| Vendor B | ISO 9001/13485 | 500+ | 2–4 weeks | ODM, IFU localization | $–$$ |
Customization and support
Clinics ask for multi-language IFUs, custom kit counts (5s/10s/25s), and branded pouches. This supplier does that, plus lot-specific COAs and export docs. Training videos are a plus, especially for new staff.
Mini case studies
Suburban clinic: Switched to Canine Corona rapid tests to separate likely viral from dietary diarrhea; reported “fewer unnecessary PCR sends” and faster client conversations.
Large kennel: During a GI flare-up, staff used Canine Corona and parvo combos for nightly screening; “helped prioritize isolation without waiting on lab couriers.”
Notes: For professional veterinary use. Negative results do not rule out infection; consider timing, shedding, and confirmatory PCR when clinically indicated.
Authoritative references
- Merck Veterinary Manual. Canine Coronavirus Infection. Accessed 2025.
- CLSI EP12-A2. User Protocol for Evaluation of Qualitative Test Performance. Clinical and Laboratory Standards Institute.
- ISO 13485:2016. Medical devices—Quality management systems—Requirements for regulatory purposes.
- WSAVA Vaccination Guidelines. World Small Animal Veterinary Association, latest update.
-
Comprehensive Guide to Syphilis Test Types: Methods, Uses, and Innovations
NewsNov.24,2025
-
Syphilis Test Strips: Affordable & Rapid Diagnostics for Global Health
NewsNov.24,2025
-
Syphilis Quick Test: Rapid Diagnosis to Improve Global Health Outcomes
NewsNov.23,2025
-
Comprehensive Guide to Syphilis Test Price: Costs, Trends, and Global Impact
NewsNov.23,2025
-
Comprehensive Guide to Syphilis Test Malaysia – Accurate, Accessible & Affordable
NewsNov.22,2025
-
Comprehensive Guide to Lab Tests for Syphilis: Global Importance and Innovations
NewsNov.22,2025

