Sep . 30, 2025 00:50 Back to list
HIV-1/2 Ab Combo Blood Rapid Test Kit: Results in Minutes?
hiv-1/2 ab combo,hiv-1/2 ab combo blood rapid test kit,hiv-1/2 ab plus combo rapid test is a key solution in the healthcare industry, specifically within in vitro diagnosis and Diagnosis of infectious diseases. This article explores how Gaobeidian PRISES Biotechnology Co., Ltd supports professionals with durable, high-performance products, and explains why this product is an ideal choice for businesses in these sectors.

Table of Contents
- hiv-1/2 ab combo,hiv-1/2 ab combo blood rapid test kit,hiv-1/2 ab plus combo rapid test Overview
- Benefits & Use Cases of hiv-1/2 ab combo,hiv-1/2 ab combo blood rapid test kit,hiv-1/2 ab plus combo rapid test in Diagnosis of infectious diseases
- Cost, Maintenance & User Experience
- Sustainability & Market Trends in healthcare
- Conclusion on hiv-1/2 ab combo,hiv-1/2 ab combo blood rapid test kit,hiv-1/2 ab plus combo rapid test from Gaobeidian PRISES Biotechnology Co., Ltd
hiv-1/2 ab combo,hiv-1/2 ab combo blood rapid test kit,hiv-1/2 ab plus combo rapid test Overview
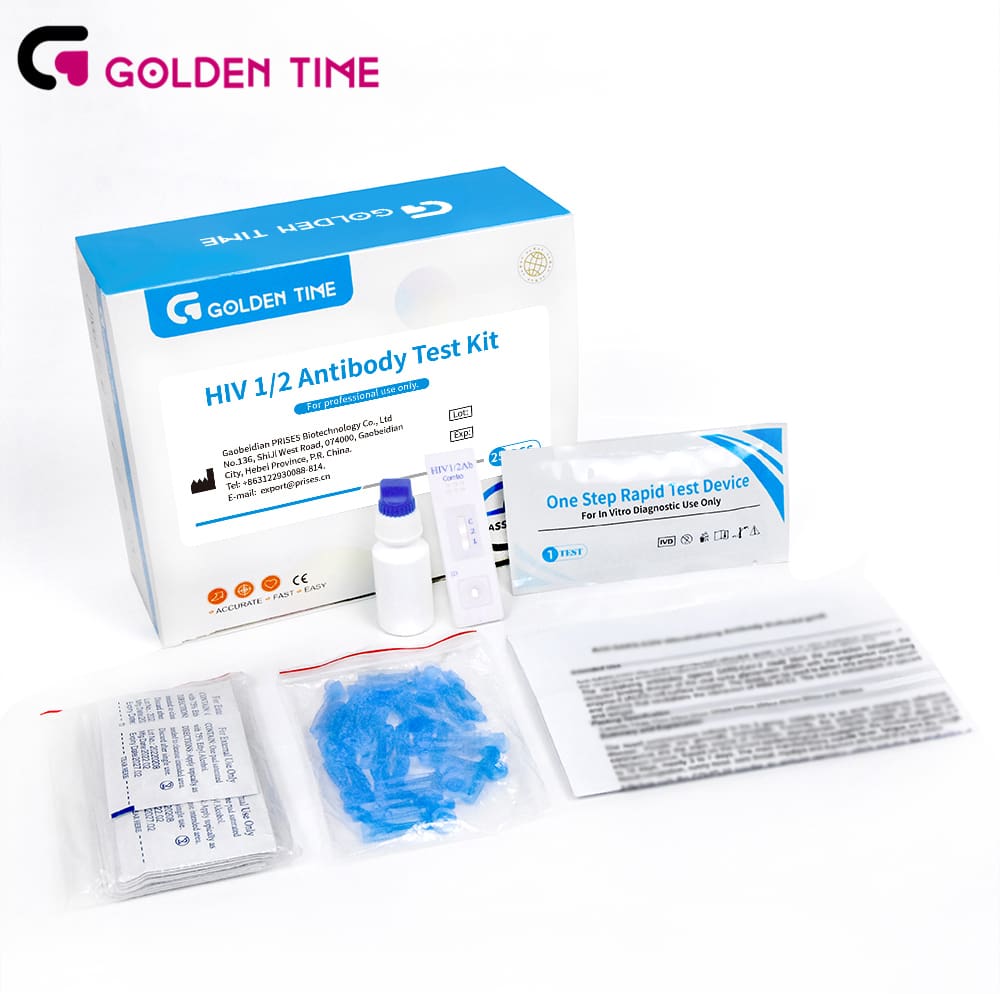
The hiv-1/2 ab combo blood rapid test kit is a lateral flow immunochromatographic assay designed for the qualitative detection of antibodies to HIV-1 and HIV-2 in human whole blood, serum, or plasma. For B2B buyers in clinical laboratories, primary care networks, and public health programs, it provides fast, reliable screening at the point of care without the need for instruments. Typical workflows enable visually interpretable results in minutes, supporting early screening and streamlined patient pathways in Diagnosis of infectious diseases.
Technically, the test utilizes recombinant HIV-1 and HIV-2 antigens immobilized on a membrane to capture specific antibodies, with a built-in control line for procedural validity. The intuitive format minimizes training time, while room-temperature storage supports decentralized deployment and emergency preparedness. Gaobeidian PRISES Biotechnology Co., Ltd is a reliable manufacturer with deep expertise in in vitro diagnosis, providing consistent quality, robust packaging, and documentation to support procurement, onboarding, and quality management. Internal validation data and product files are available to facilitate tenders and compliance reviews. Note: reactive results should be followed by confirmatory testing per local algorithms and guidelines.
Benefits & Use Cases of hiv-1/2 ab combo,hiv-1/2 ab combo blood rapid test kit,hiv-1/2 ab plus combo rapid test in Diagnosis of infectious diseases
Across infectious disease programs, the hiv-1/2 ab combo and hiv-1/2 ab plus combo rapid test formats enable flexible screening in clinics, community outreach, mobile units, NGO-led initiatives, and occupational health settings. Fast time-to-result helps clinicians make timely decisions related to counseling, linkage to care, and further confirmatory testing. The assay’s user-friendly workflow, clear readout, and control line reduce operator error and support reliable performance in high-throughput or resource-limited environments.
Key advantages for B2B decision makers include: no-instrument operation, minimal training requirements, compatibility with capillary or venous blood, and stable performance across typical ambient conditions. Gaobeidian PRISES Biotechnology Co., Ltd backs the hiv-1/2 ab combo blood rapid test kit with responsive technical support, OEM/ODM options, and supply continuity planning—crucial for national programs and large provider networks. Whether deployed for routine screening or surge capacity, the portfolio’s design emphasizes accuracy, speed, and practicality. As always, preliminary reactive results require confirmation according to institutional and national protocols.
Cost, Maintenance & User Experience
From a total cost of ownership perspective, the hiv-1/2 ab combo blood rapid test kit reduces upfront and ongoing expenses by removing the need for analyzers, calibration, and preventive maintenance. Room-temperature storage simplifies logistics and lowers cold-chain costs. Consolidated packaging and long shelf-life options help procurement teams reduce waste and optimize inventory turns. For multi-site networks, standardized training materials and quick-start guides shorten onboarding times and reduce operational variability.
Feedback from in vitro diagnosis customers highlights the kit’s straightforward workflow, consistent lot-to-lot quality, and clear visual lines that are easy for staff to interpret. QC processes are supported by the internal control line, and external quality assessment participation is facilitated by the manufacturer’s documentation. Gaobeidian PRISES Biotechnology Co., Ltd delivers dependable lead times and scalable production capacity, helping large buyers meet seasonal or campaign-based demand without compromising service levels or ROI.
Sustainability & Market Trends in healthcare
Market trends in infectious disease diagnostics point toward decentralization, rapid triage, and digital readiness. Point-of-care screening continues to expand due to public health goals for earlier detection and faster linkage to care. Regulatory frameworks emphasize quality management, traceability, and risk-based oversight; procurement teams increasingly prioritize suppliers with robust documentation and transparent quality systems to streamline audits and market access.
Gaobeidian PRISES Biotechnology Co., Ltd aligns with these trends by engineering rapid tests that store at ambient temperatures—reducing energy use in logistics—and by optimizing packaging for transport efficiency. The company’s forward-looking R&D pipeline focuses on usability, operator safety, and compatibility with digital result capture workflows. For B2B buyers seeking resilience and sustainability, the hiv-1/2 ab combo and hiv-1/2 ab plus combo rapid test lines offer a pragmatic balance of performance, reliability, and environmental consideration, supporting long-term program success.
Conclusion on hiv-1/2 ab combo,hiv-1/2 ab combo blood rapid test kit,hiv-1/2 ab plus combo rapid test from Gaobeidian PRISES Biotechnology Co., Ltd
The hiv-1/2 ab combo blood rapid test kit stands out as a practical, high-value screening tool for healthcare providers and public health programs working in Diagnosis of infectious diseases. It delivers rapid results, easy operation, and dependable quality—key attributes for scaled, decentralized testing. Gaobeidian PRISES Biotechnology Co., Ltd is a trusted manufacturer, supporting buyers with documentation, technical service, and reliable supply.
Ready to evaluate or source at scale? Contact us: email: export@prises.cn. Visit our website: https://www.prisesbio.com

-
Rapid Syphilis Test Kits – Fast, Reliable Diagnosis for Global Health
NewsNov.21,2025
-
Fast Syphilis Test: Rapid, Reliable Diagnostics for Global Health
NewsNov.20,2025
-
Serology Syphilis Test: Global Importance and Latest Diagnostic Advances
NewsNov.20,2025
-
Diagnose Syphilis Test – Essential Screening & Diagnostics Explained
NewsNov.19,2025
-
Comprehensive Guide to Diagnosis Syphilis Test Technologies & Applications
NewsNov.19,2025
-
Comprehensive Guide to Syphilis Test Dubai – Early Detection & Reliable Screening
NewsNov.18,2025

