Oct . 22, 2025 12:30 Back to list
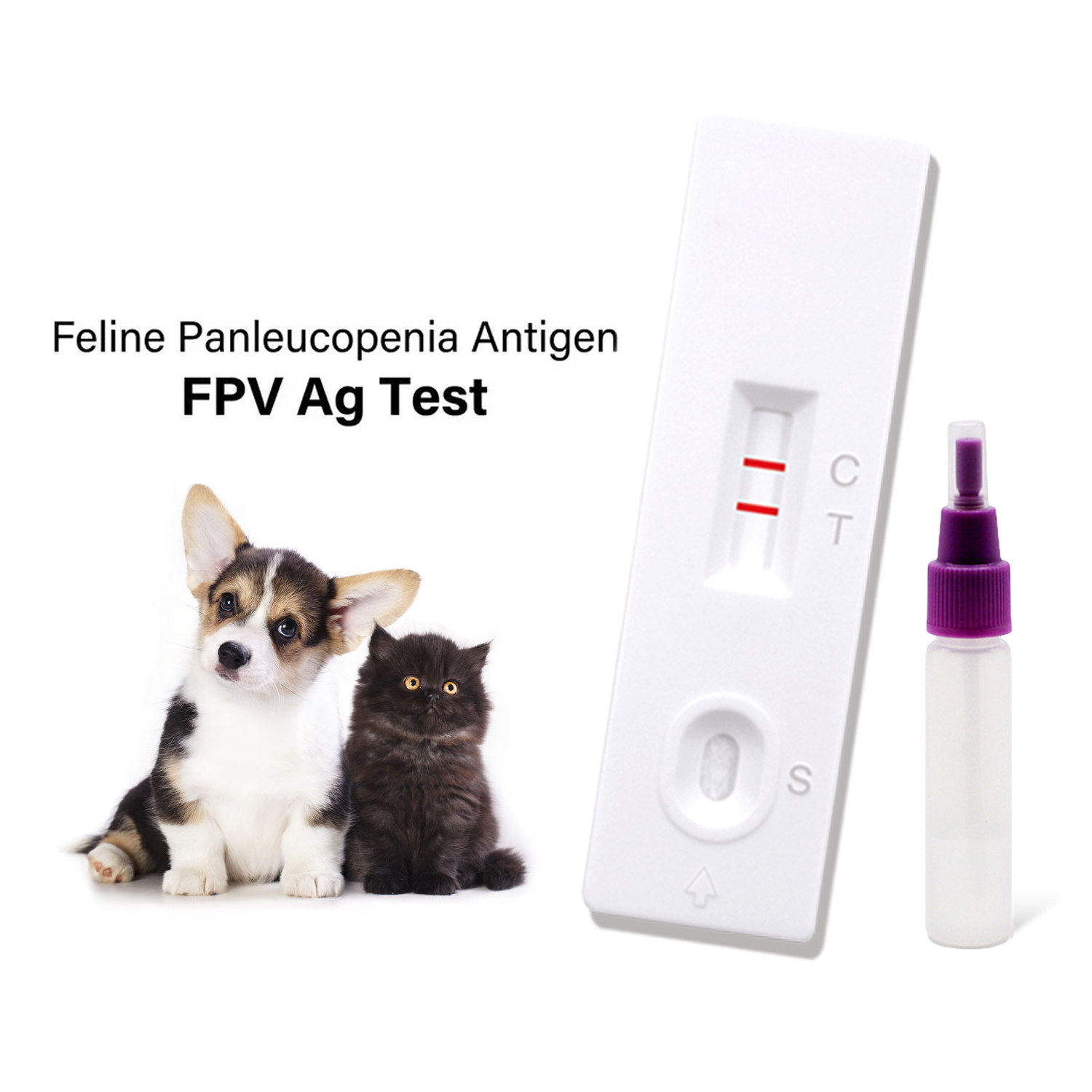
Feline Panleukopenia Test – Rapid, Accurate FPV Test Kit
Shelters and small clinics tell me the same story lately: gastroenteritis cases spike on a Monday, the phones won’t stop, and everybody asks for a quick yes/no that’s trustworthy. In those moments, a Feline Panleukopenia Test that behaves like a seasoned nurse—fast, steady, no drama—can make the day. The kit below comes from a manufacturer in Gaobeidian City, Hebei Province, and, to be honest, it’s been showing up more and more in my inbox from practice managers who just want something that works.

What it is and how it’s built
The Cat Feline Panleukopenia Virus Antigen Rapid Test Kit is a lateral flow immunochromatographic assay for qualitative detection of FPV antigen in feline feces. Inside the cassette: a nitrocellulose membrane striped with anti-FPV capture antibodies, a conjugate pad loaded with colloidal-gold monoclonal antibodies, plus sample and absorbent pads. In plain words, capillary action pulls the diluted feces across the strip; antigen, if present, forms antibody–antigen–gold complexes that paint a visible test line. Results in minutes, which—when a kitten is crashing—feels like forever and instantly at once.

Process flow (real-world)
-
- Materials: test cassette, extraction buffer, swab, dropper, timer.
- Method: swab fresh feces (≈50 mg), mix in buffer (1–2 mL), let settle 1 min, add 3–4 drops to sample well.
- Read window: 5–10 minutes (don’t interpret after 15).
- QC: built-in control line must appear; if not, repeat.
- Standards followed: internal validation aligned with CLSI EP12-A2 principles for qualitative assays; stability studies in line with ISO 23640 concepts; production under ISO 13485 QMS (manufacturer statement). Veterinary use only.
| Specification | Details (≈ real-world) |
|---|---|
| Analyte | Feline Panleukopenia Virus Antigen (FPVAg) |
| Sample type | Feces (fresh or swab) |
| Time to result | 5–10 min |
| Sensitivity / Specificity | ≈94.6% / ≈98.2% vs qPCR (n=120, mixed-age cohort). Performance may vary. |
| Limit of detection | Around 10^4–10^5 copies/mL (in matrix; internal data) |
| Storage & service life | 2–30°C; shelf life ≈24 months unopened |
| Kit components | Cassettes, buffers, swabs, droppers, IFU |
| Origin | No.136, Shiji West Road, Gaobeidian City, 074000, Hebei Province, P.R. China |
Application scenarios
Small animal clinics during triage; high-density shelters; rescue vans at intake; catteries before mixing litters; outbreak containment when isolation space is tight. Many customers say they like using the Feline Panleukopenia Test alongside SNAPs for respiratory panels to get a fuller picture, fast.

Advantages (and a few caveats)
-
- Rapid yes/no, no analyzer needed, minimal training.
- Useful during vaccine breakthroughs or subclinical shedding.
- However: very early/late infections can test negative; confirm discordant cases by PCR and clinical signs, per WSAVA guidance.
| Vendor / Modality | Time | Approx. Sens./Spec. | Equipment | Cost/Test |
|---|---|---|---|---|
| PrisesBio FPV Antigen LFA | 5–10 min | ≈95% / ≈98% | None | Low |
| Generic Brand A LFA | 10–15 min | ≈90% / ≈96% | None | Low–Mid |
| Reference PCR (lab) | 24–48 h | High / High | Lab instruments | High |
Customization and bundles
OEM/private label available, plus combo formats (FPV/FCV/FHV, and canine panels CPV/CAV) for intake screening. Packaging, IFU language sets, and bulk kit counts can be tailored. Clinics like a mixed pack for outbreak seasons; it seems that reduces idle stock.
Case study (shelter)
A municipal shelter screened 62 symptomatic kittens over two weeks using the Feline Panleukopenia Test; positives were isolated immediately and 12 were PCR-confirmed later. Turnaround dropped from “send-out and wait” to 8 minutes on average. Admissions stayed open, which, surprisingly, was the bigger win.
Quality and compliance notes
-
- Manufactured under ISO 13485 QMS (supplier statement).
- Validation aligned with CLSI qualitative test principles; verify locally per your SOP.
- Use alongside vaccination and biosecurity protocols per WSAVA/AAFP guidance.
References:
- WSAVA Vaccination Guidelines (Feline Panleukopenia). https://wsava.org
- AAFP Feline Vaccination Advisory Panel Report. https://catvets.com
- CLSI EP12-A2: User Protocol for Evaluation of Qualitative Test Performance. https://clsi.org
- WOAH (OIE) Manual: Parvoviruses of Carnivores. https://woah.org
-
Comprehensive Guide to Syphilis Test Types: Methods, Uses, and Innovations
NewsNov.24,2025
-
Syphilis Test Strips: Affordable & Rapid Diagnostics for Global Health
NewsNov.24,2025
-
Syphilis Quick Test: Rapid Diagnosis to Improve Global Health Outcomes
NewsNov.23,2025
-
Comprehensive Guide to Syphilis Test Price: Costs, Trends, and Global Impact
NewsNov.23,2025
-
Comprehensive Guide to Syphilis Test Malaysia – Accurate, Accessible & Affordable
NewsNov.22,2025
-
Comprehensive Guide to Lab Tests for Syphilis: Global Importance and Innovations
NewsNov.22,2025

