Tag/nasal test swab nylon flocked swabs
-
 COVID-19 (SARS-CoV-2) IgG IgM Antibody Test
COVID-19 (SARS-CoV-2) IgG IgM Antibody Test -
 COVID-19 (SARS-CoV-2) Antigen Test Kit
COVID-19 (SARS-CoV-2) Antigen Test Kit -
 COVID-19 (SARS-CoV-2) Neutralizing Antibody Test
COVID-19 (SARS-CoV-2) Neutralizing Antibody Test -
 COVID-19 (SARS-CoV-2) Antigen Diagnostic Test Kit
COVID-19 (SARS-CoV-2) Antigen Diagnostic Test Kit -
 HCG Pregnancy Test Midstream
HCG Pregnancy Test Midstream -
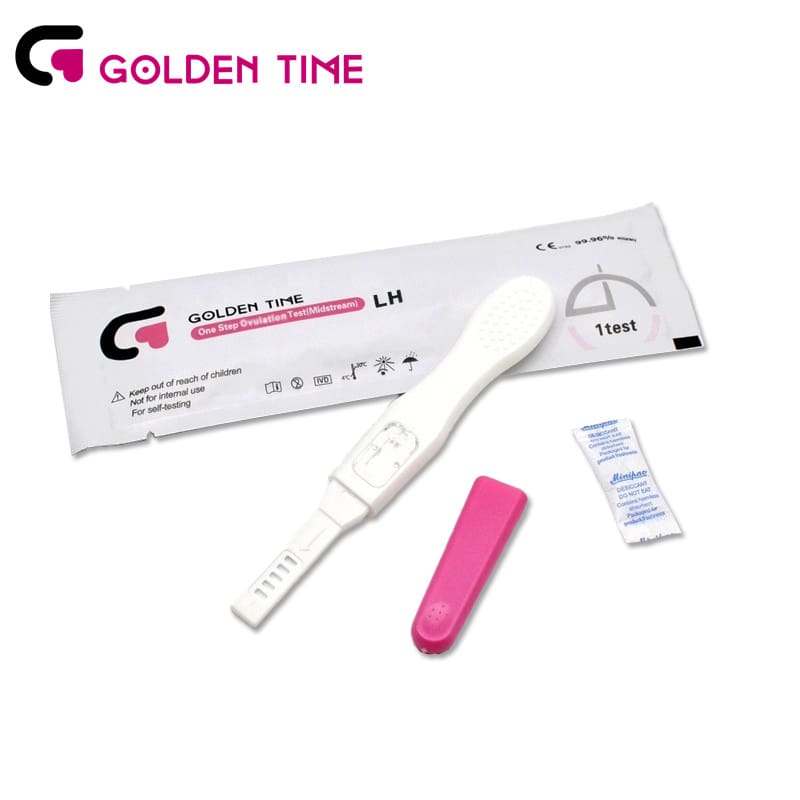
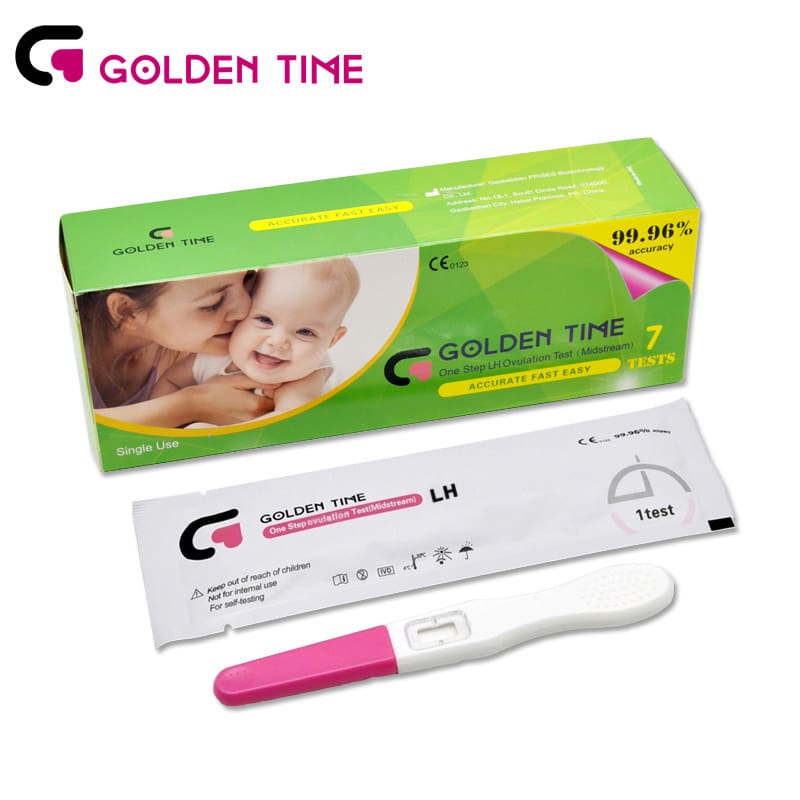 Urine LH Ovulation Test Midstream
Urine LH Ovulation Test Midstream -
 LH Ovulation Test Strip
LH Ovulation Test Strip -
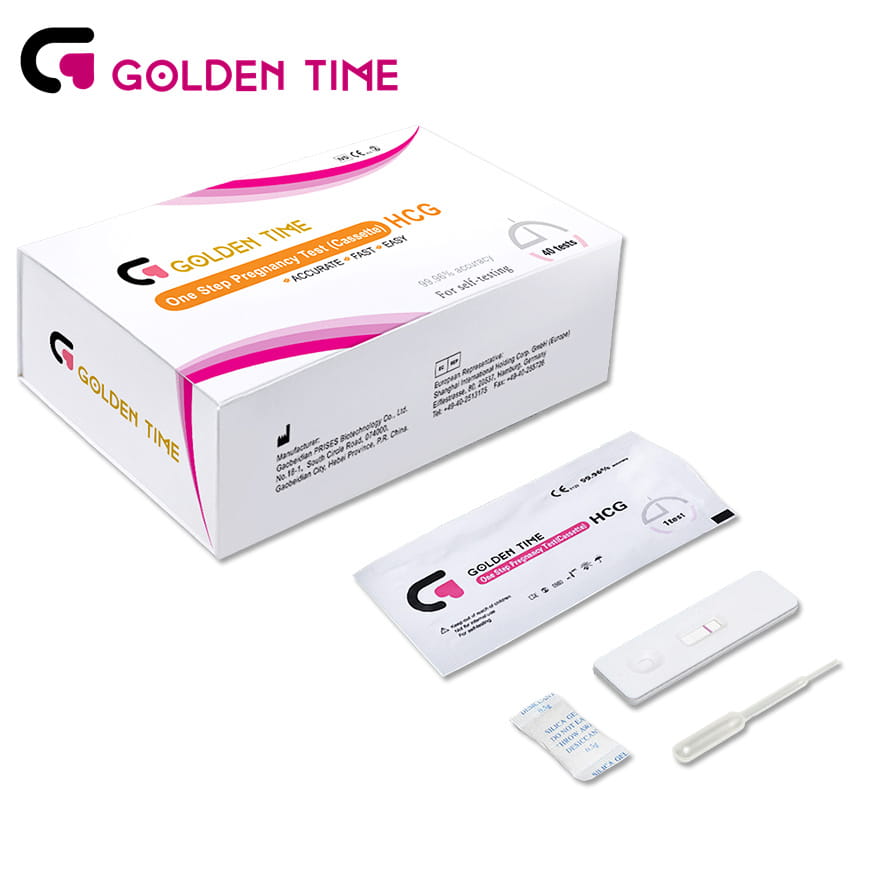 40 Pieces Home Early One Step Pregnancy Test Cassette
40 Pieces Home Early One Step Pregnancy Test Cassette -
 Abnormal Pregnancy Screening Test Kit
Abnormal Pregnancy Screening Test Kit -
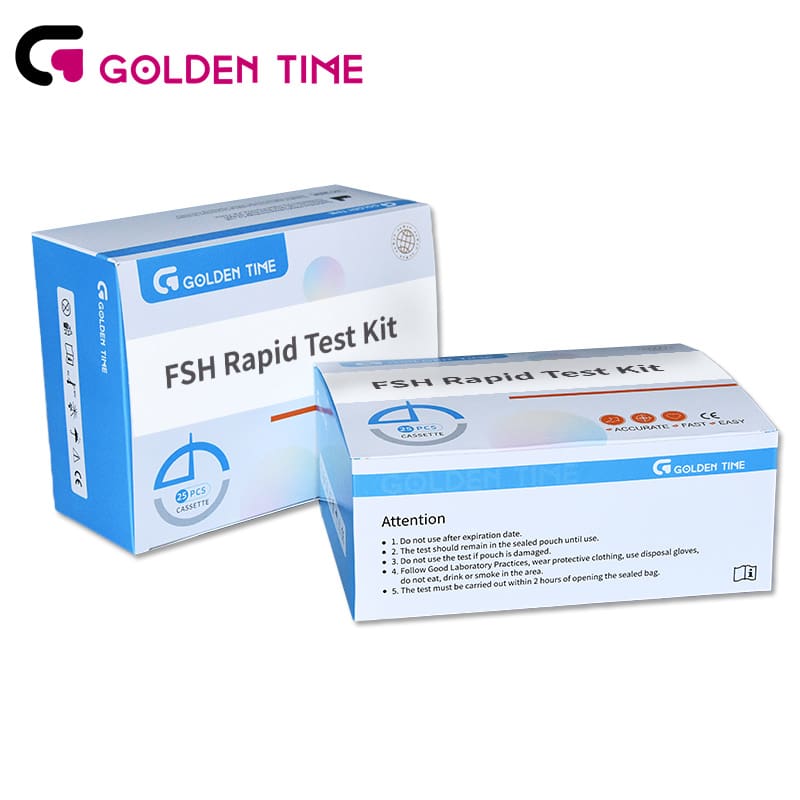 Home Follicle Stimulating Hormone FSH Test Kit
Home Follicle Stimulating Hormone FSH Test Kit
Produtct Title
nasal test swab nylon flocked swabs-
Urine HCG Pregnancy Test Strip
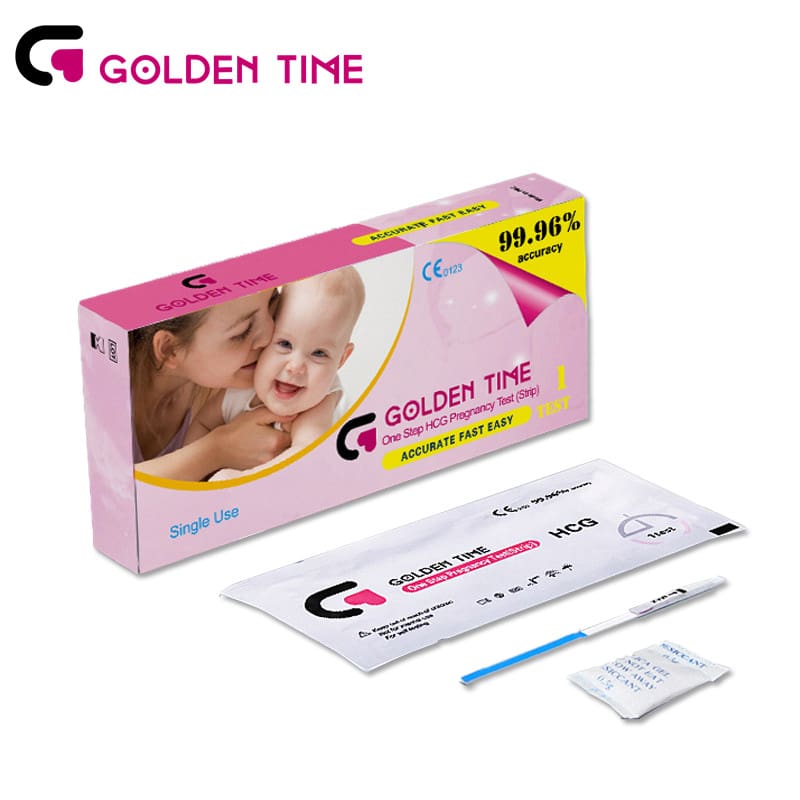
-
HCG Pregnancy Test Cassette
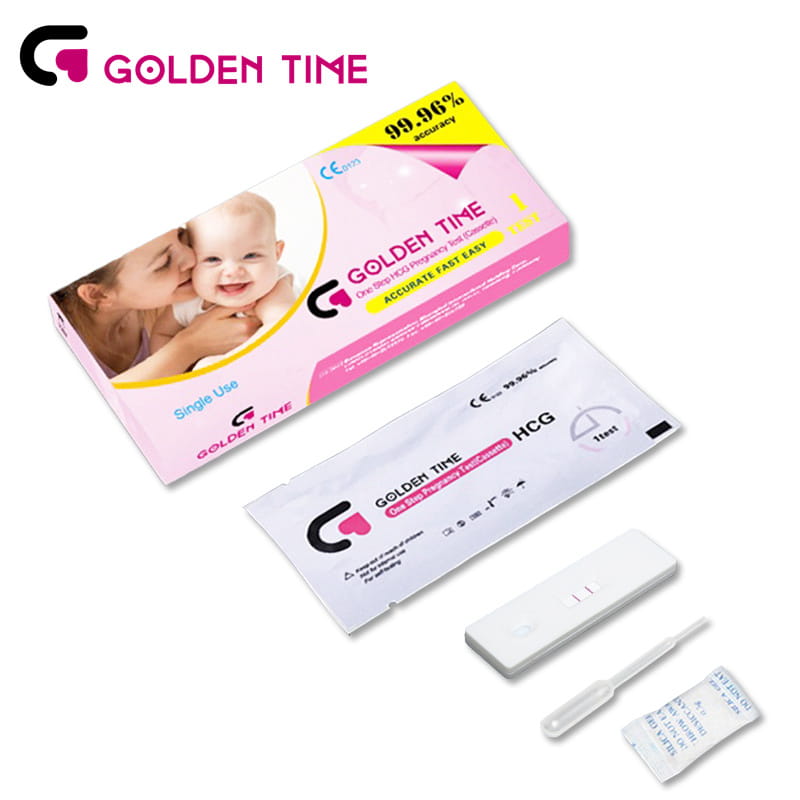
-
Cardiac Marker C-reactive Protein (CRP) Rapid Test Kit

-
Cardiac Troponin i (TRO) Rapid Test Kit

-
Cardiac Marker Myoglobin (MYO) Rapid Test Cassette

-
Carcinoembryonic Antigen (CEA) Rapid Test Cassette

-
Cardiac Marker CK-MB Rapid Test Cassette

-
Prostate Specific Antigen (PSA) Rapid Test Cassette

-
Tumor Marker Alpha-Fetoprotein (AFP) Rapid Test Cassette

-
FOB Fecal Occult Blood Rapid Test Kit

-
Dengue Antibody IgG/IgM Combo Blood Rapid Test Kit

-
Sterile Throat Swab Nylon Flocked

-
Chlamydia Trachomatis CT Antigen Rapid Test Kit
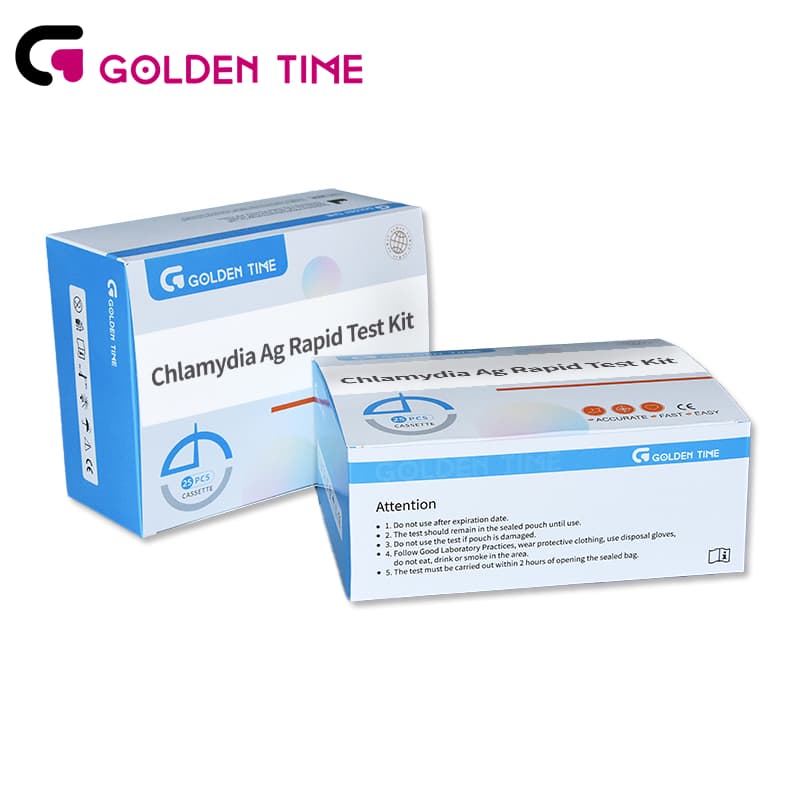
-
hCG One-Step Pregnancy Weeks Test
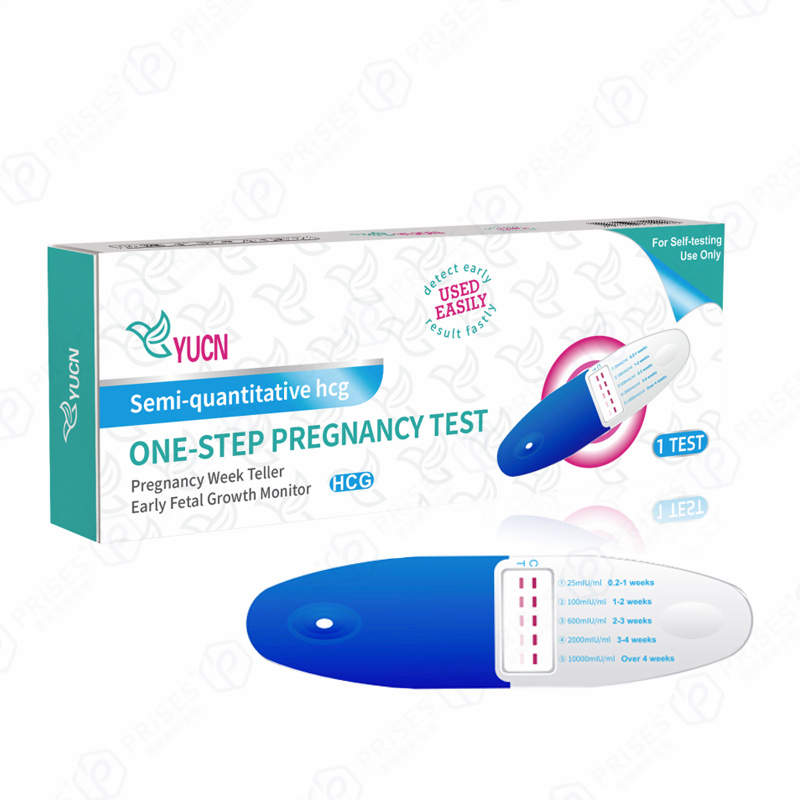
Related News
-
2025-01-13NEW PRODUCT —— COVID-19 (SARS-CoV-2) Swab Antigen Diagnostic Rapid Test KitPRISES 2019-nCoV Antigen Test is a lateral flow assay for self-tested by professional to aid diagnosis of COVID-19 infection with a nasal swab, delivering results in as little as 15 minutes without an instrument. The test can not only provide users high flexibility to use in all types of settings...
-
2025-01-13Golden Time COVID-19 antigen rapid test and neutralizing antibody test for professional use got a White List from just nowCongratulations!!!Golden Time COVID-19 antigen rapid test and neutralizing antibody test for professional use got a White List from just now. Moreover, COVID-19 Antigen Rapid Test had also successfully registered in many countries!THANKS to our colleagues across Prises Biotech for the treme...
-
2024-02-27Rapid test-What is a pregnancy test?Home pregnancy tests (also called HPTs) detect human chorionic gonadotropin (hCG), a hormone produced during pregnancy. When a fertilized egg implants into your uterine wall, the placenta begins to grow, releasing hCG into your bloodstream. Some home pregnancy tests can detect hCG in your urine six days before your missed period. For example, with the Clearblue® Early Detection Pregnancy Test, 71% of pregnancies can be detected 6 days before the missed period (5 days before the expected period).> Calculate when I can test
-
2024-02-27Pregnancy test-What Is a Pregnancy Test?A pregnancy test checks your pee or blood to see if you're pregnant.There are different reasons you might take a pregnancy test. Maybe you're trying to get pregnant and you want a positive result. Or maybe something interrupted your birth control. You also might take a pregnancy test before having a medical procedure or starting a new medication, to avoid complications. This is true whether you're biologically female, nonbinary, or transgender male. If you have a uterus and ovaries (and you're ovulating), and you’re having penetrative sex with a penis, you can get pregnant.Here are the answers to some common questions about pregnancy tests.
-
2024-02-27When to take a home pregnancy test-How early can home pregnancy tests show positive results?Home pregnancy tests can be very accurate if used properly.Since the earliest recorded history, women have had a strong desire to know whether they are pregnant as early as possible. The body goes through countless changes in the first trimester, and one of the first indicators is a change in the hormones that leave the body through urine.Ancient Egyptians relied on a form of urine testing to determine pregnancy status way back in 1350 BCE. A woman urinated daily on wheat or barley seeds and if the plants grew, it meant she was pregnant. Modern-day validation suggests that test was about 70% accurate in detecting human chorionic gonadotropin (hCG), a hormone produced by a woman’s body soon after implantation of a fertilized egg inside the uterus.
-
2024-02-27Pregnancy test-When Can I Take a Pregnancy Test?The Best Time of Day and the Best Time in Your CycleFor many people, the decision of when to take a pregnancy test can be a major source of anxiety. Sometimes the stress is because you want to be pregnant. Sometimes it’s because you don’t want to be.Regardless of whether you’re hoping for a negative or a positive result, taking an early test may seem like a good way to find out if you’re pregnant right away. Unfortunately, early testing may give you a negative result, even if you are pregnant.It's important to take the test at the right time to maximize your chance of getting an accurate reading. Learn the best time to take a pregnancy test in order to get the most accurate result, as well as the risks associated with testing too early.
-
2024-02-27Rapid test-How long does it take to know I’m pregnant?Step back from the supermarket shelves; you should wait until the first day of a missed period before you do a pregnancy test. This is usually about two weeks after you think you conceived. So get your diary out and start counting.Too keen to wait? Some tests are more sensitive than others and can be taken earlier. This might be four or five days before your period is due.If you can though, it’s most reliable to wait for the first day of your missed period. And that way you won’t get any upset from an inaccurate result.
-
2024-02-27Pregnancy test-When you can do a pregnancy testYou can buy pregnancy testing kits from pharmacists and some supermarkets. They can give a quick result and you can do the test in private.The following places provide free pregnancy tests:sexual health servicessome young people's services – call the national sexual health helpline on 0300 123 7123 for detailsBrook centres for under-25s – find your nearest Brook centreYou may also be able to get a pregnancy test free of charge from your GP.
-
2024-02-27Pregnancy test-When should I take a pregnancy test?From the very beginning of pregnancy, your body starts to go through changes to support the cells that will develop into your baby. One thing that happens very quickly is the production of HCG. If you’re pregnant, your body starts to produce more HCG. Your HCG levels start to build up once the fertilized egg implants in your uterus — about six to 10 days after conception.There are two main types of pregnancy tests — urine tests and blood tests. Often, you’ll take a urine test at home with a home pregnancy test. This type of test is available over the counter (you don’t need a prescription from your healthcare provider) and in a variety of price ranges. Blood tests to check for pregnancy happen in your healthcare provider’s office and involve giving a sample of your blood. The other way to confirm a pregnancy is by using an ultrasound. Your provider performs an ultrasound in their office.
-
2024-02-27Pregnancy test-What is a pregnancy test?A pregnancy test can tell whether you're pregnant by checking a sample of your urine (pee) or blood for a specific hormone. The hormone is called human chorionic gonadotropin (hCG). High levels of hCG are a sign of pregnancy. hCG increases quickly in the first ten weeks after a fertilized egg attaches to the inside wall of the uterus.Urine tests for pregnancy are most accurate when you do the test a week or two after you've missed your menstrual period. If you take a urine test too close to the time you got pregnant, the test could say that you are not pregnant even when you really are. That's because your body may not yet have made enough hCG to show up on the test.You can have an hCG urine test at your health care provider's office or you can do the test yourself with an at-home test kit. These tests are basically the same, so many people use a home pregnancy test before calling their provider. If you follow the instructions carefully, home pregnancy tests are about 97-99% accurate. They can give you the results in minutes.
-
2024-03-18All You Need to Know About the Widal Test for Typhoid FeverA widal test report is more commonly known as the typhoid test report, as the widal test is done for diagnosing typhoid fever. Typhoid fever is a bacterial infection caused by the bacterium Salmonella Typhi, and it is commonly spread through contaminated food or water.
-
2024-03-18What Is Typhoid Fever and How to Use the Typhoid Rapid Test Kit?Responsible for more than 9 million cases and 110,000 deaths worldwide, typhoid fever remains a major threat to public health across the developing regions of the world. According to the World Health Organization (WHO), typhoid fever is endemic in many parts of Asia, Africa, and Latin America. It disproportionately affects children and young adults in communities with inadequate sanitation and limited access to clean water.
-
2024-03-18What is the Typhidot Test?A Typhidot Test is a screening test for typhoid fever performed using a blood sample. Typhoid fever, also called enteric fever, is a bacterial infection caused by Salmonella typhi. Typhoid fever is one of the most common bacterial infections in low-income nations, including India.
-
2024-03-18Here's what a Widal Test Positive means for someone with FeverIf you are experiencing the symptoms of typhoid fever and you have a 103F fever then you need to get a widal test done. The widal test is a preliminary analysis to diagnose typhoid fever in people, and a positive report means that you may have the bacteria.
-
2024-03-18What is the rapid test method for syphilis?Syphilis is a dangerous disease caused by the spirochete Treponema pallidum (spirochete syphilis). The disease causes serious complications on nerves, eyes, skin and mucous membranes if not detected and treated promptly.
-
2024-03-20Rapid test-How Soon After Unprotected Sex Should You Take a Pregnancy Test?Brandi Jones has over two decades of experience as a nurse in an acute care setting. Her clinical background includes pediatrics, medical-surgical, and women's health. She also specializes in professional staff development.HEALTH'S EDITORIAL GUIDELINES If you've recently had unprotected sex, it's common to wonder when you should test for pregnancy. The short answer: home pregnancy tests are most accurate when you take them after your period is late. Generally, you can take early-detection pregnancy tests eight days after conception. However, experts suggest that you'll get your most accurate test results when you take a test 10 to 14 days after unprotected sex.1
-
2024-03-20Pregnancy test-12 Early Signs of PregnancyWhen you have decided that you’re ready for a baby, sometimes waiting to see the two little lines on a pregnancy test can feel like forever. Will it be this month? When you’re trying to conceive, you may overanalyze any new feeling that you’re experiencing. Are you bloated? Sore breasts? Is your lunch just not sitting right on your stomach?We break down the most common early signs of pregnancy and what you can do to feel more comfortable while your little bundle of joy is growing.
-
2024-03-20Medical devices-How Soon After Sex Can You Take a Pregnancy Test?If you’re wondering if you’re pregnant, you need an answer. Immediately. However, that’s not necessarily possible if you want the most accurate results.How soon can you take a pregnancy test after sex? The answer may surprise you.“Many women panic when they realize they’ve missed a birth control pill or they’ve missed a period,” said Dr. Hardison. “We want you to know that we can help guide you through this process in a way that ensures you’re getting the most accurate results.”
-
2024-03-20Rapid test-How Soon After Unprotected Sex Can You Test for Pregnancy?Whether you're actively trying to get pregnant or not, the time between unprotected sex and when you can take a pregnancy test feels like forever. But if you test too soon, you risk getting a false-negative result, meaning you're pregnant but the test shows you're not.1The right time to take a pregnancy test after having unprotected sex is the first day after a missed period or 21 days after unprotected sex if you have irregular periods.This article will explain why it's important to wait and how to take a pregnancy test for accurate results.
-
2024-03-20Pregnancy test-When can I take a pregnancy test?After the egg is fertilized, it travels to the uterus (womb) and begins to implant in the uterine wall. If implantation is successful, tiny amounts of the pregnancy hormone, hCG, can start to appear in your urine from around 7 – 9 days after ovulation. It is this hormone that all home pregnancy tests detect. Some early pregnancy tests, such as the Clearblue Early Detection Pregnancy Test, can tell you whether you are pregnant as early as 6 days before your missed period (5 days before you expect your period)1.
Related Search
- covid quick test
- test covid 19
- covid test
- test for covid-19
- covid- 19 test
- test covid
- covid 19 ag test
- self test covid
- covid self test
- self test covid 19
- test kit covid
- covid 19 test kid
- covid rapid test
- rapid test for covid-19
- covid 19 rapid test
- covide-19 test rapid
- rapid test covid
- rapid covid test
- rapid test covid 19
- covid 19 test kit home
- covid saliva test
- covid test rapid diagnostic
- covid 19 antigen test
- covid antigen test
- antigen test covid
- covid 19 rapid antigen test
- covid antigen rapid test
- covid-19 antigen rapid test
- antigen rapid test covid
- sars-cov-2 antigen rapid test
