Oct . 16, 2025 14:25 Back to list
Lateral Flow Plastic Cassette | Durable, OEM, Tight Seal
Inside the humble lateral-flow housing: the FOB Fecal Occult Blood Rapid Test Cassette
Every few months I tour a diagnostics line, and I’m reminded how much work hides inside a small plastic shell. The lateral flow plastic cassette for the FOB Fecal Occult Blood Rapid Test Kit is a good example—simple on the outside, a tight choreography of membranes, antibodies, and manufacturing discipline on the inside.

Industry pulse
Rapid diagnostics are shifting from lab-centric to near-patient settings—physicians’ offices, community clinics, even mobile units. For FIT/FOB screening in particular, speed and simplicity are everything. Manufacturers that prove lot-to-lot consistency and practical usability win tenders; to be honest, buyers these days are also probing sustainability and private-label flexibility more than ever.

Materials and build (what’s inside)
- Housing: polystyrene lateral flow plastic cassette, snap-fit with sample and result windows.
- Sample pad: cellulose or glass-fiber, pretreated to reduce matrix effects.
- Conjugate pad: anti-human hemoglobin antibodies labeled with colloidal gold.
- Nitrocellulose membrane: test and control lines striped with capture antibodies.
- Absorbent pad: wicks flow; foil pouch with desiccant controls moisture.
Product snapshot: FOB Fecal Occult Blood Rapid Test (Colloidal Gold)
| Intended use | Qualitative detection of fecal occult blood in laboratories or physicians’ offices |
| Format | lateral flow plastic cassette, colloidal gold immunoassay |
| Specimen | Stool sample diluted in buffer |
| Time to result | ≈ 5–10 minutes (real-world use may vary) |
| Analytical sensitivity | Around 50 ng/mL human hemoglobin equivalence, typical for FIT cassettes |
| Shelf life | 18–24 months sealed at 2–30°C (per ISO 23640 principles) |
| Certifications | Manufactured under ISO 13485; risk managed per ISO 14971 |
| Origin | No.136, Shiji West Road, Gaobeidian City, 074000, Hebei, P.R. China |
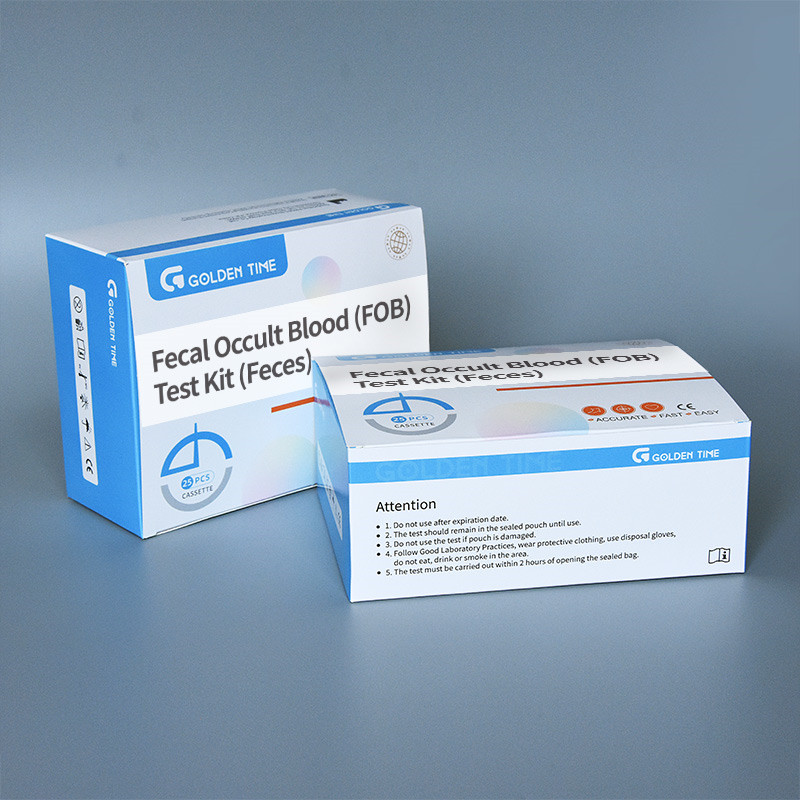
Method overview and validation
Procedure is straightforward: collect stool with the provided device, vortex or mix in buffer, add drops to the sample well, and read within the indicated window. Analytical verification typically follows CLSI EP12-A2 for qualitative tests, with stability validated along ISO 23640. Many buyers ask about lot traceability—good vendors keep membrane roll maps, conjugate batch IDs, and incoming QC certs. It sounds nerdy, but it matters for recalls and audits.
Where it’s used
- Physicians’ offices and GI clinics for quick triage
- Small clinical labs needing cost-effective FIT screening
- Outreach programs where compact lateral flow plastic cassette kits travel well

Vendor comparison (indicative)
| Vendor | Certs | Customization | MOQ | Lead time |
|---|---|---|---|---|
| Prises Bio (FOB FIT) | ISO 13485; design files available for audit | Cassette color, labeling, IFU languages, barcodes | ≈ 5k–10k | ≈ 3–5 weeks (forecast-driven) |
| Generic A | ISO 13485 | Label + pouch only | ≈ 20k | 6–8 weeks |
| Generic B | ISO 13485, CE IVD listed | Broader, but higher tooling fees | ≈ 10k | 4–6 weeks |
Note: values are indicative; real-world use may vary by forecast and region.
Customization and practical tips
- Private label: pouch, cassette emboss, IFU layout.
- Cassette geometry: single vs. dual window; dropper tip calibration.
- Packaging: 1T, 5T, 20T kits; desiccant size tuned to climate.
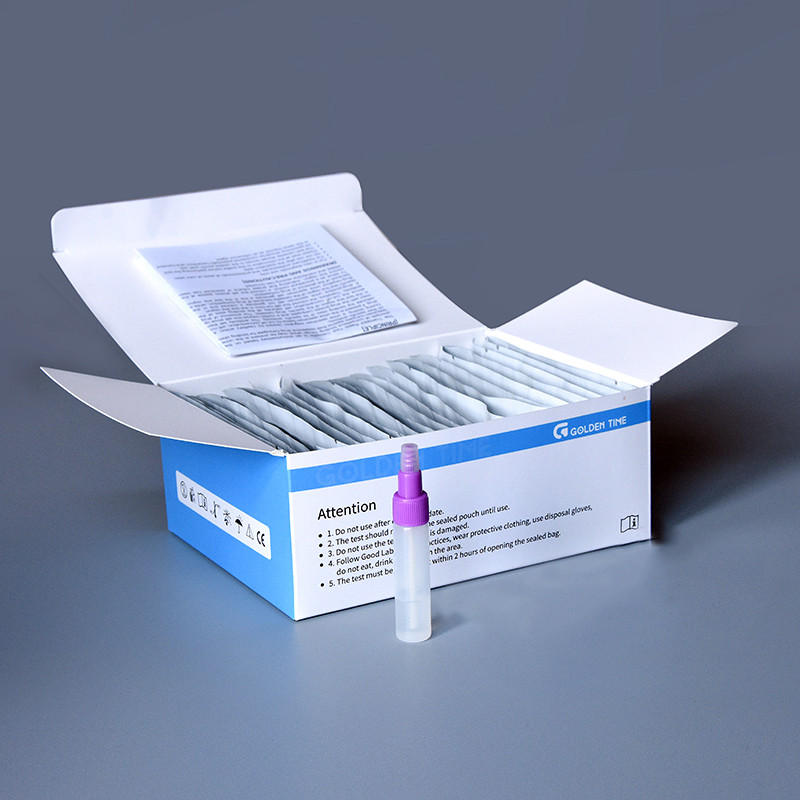
Case notes and feedback
A clinic network in Southeast Asia rolled out 15,000 kits over a quarter; they reported fewer invalids after switching to buffered collection vials with clearer fill lines. Another lab director told me, “control lines are crisp even with somewhat mucky samples,” which, admittedly, is what you want to hear in FOB testing.
Standards and compliance at a glance
- ISO 13485 quality management for IVDs
- ISO 14971 risk management
- CLSI EP12-A2 for qualitative method evaluation
- ISO 23640 stability studies for IVD reagents
- WHO guidance on POCT implementation and training
References:
- CLSI. EP12-A2: User Protocol for Evaluation of Qualitative Test Performance.
- ISO 13485:2016 Medical devices—Quality management systems—Requirements for regulatory purposes.
- ISO 23640:2015 In vitro diagnostic medical devices—Evaluation of stability.
- ISO 14971:2019 Medical devices—Application of risk management.
- WHO. Point-of-care testing: principles and operational guidance.
-
Comprehensive Guide to Syphilis Test Types: Methods, Uses, and Innovations
NewsNov.24,2025
-
Syphilis Test Strips: Affordable & Rapid Diagnostics for Global Health
NewsNov.24,2025
-
Syphilis Quick Test: Rapid Diagnosis to Improve Global Health Outcomes
NewsNov.23,2025
-
Comprehensive Guide to Syphilis Test Price: Costs, Trends, and Global Impact
NewsNov.23,2025
-
Comprehensive Guide to Syphilis Test Malaysia – Accurate, Accessible & Affordable
NewsNov.22,2025
-
Comprehensive Guide to Lab Tests for Syphilis: Global Importance and Innovations
NewsNov.22,2025

