Jan . 09, 2025 10:40 Back to list
COVID-19 (SARS-CoV-2) IgG IgM Antibody Test
Navigating the complex world of COVID-19 testing can be daunting, but understanding the nuances is crucial in today's healthcare landscape. The advent of the pandemic has intensified the need for reliable and efficient testing methods, enabling timely diagnosis and treatment. This guide seeks to illuminate various facets of COVID-19 testing, grounding its insights in authentic experiences and authoritative data, thereby fostering trust among readers.
Expertise from virologists and laboratory technicians has been invaluable in refining testing techniques. Enhanced methodologies, such as multiplex PCR that targets multiple sections of the virus’s genome, reduce false negatives and increase reliability. Collaborative efforts between medical institutions and biotech firms have accelerated technological advancements, ensuring rapid test development and efficient distribution. The role of authoritative bodies cannot be overstated. Institutions like the World Health Organization (WHO), the Centers for Disease Control and Prevention (CDC), and the Food and Drug Administration (FDA) provide stringent guidelines for test approval and use, safeguarding public health. Their ongoing research and data dissemination play a pivotal role in educating the public and health professionals, reinforcing trust in testing methodologies. Trustworthy information is the backbone of effective understanding and implementation of COVID-19 testing protocols. Reliable data from peer-reviewed studies and real-world applications offer insights into testing effectiveness and potential pitfalls. Regular updates from government and health authorities help dispel myths and encompass emerging variants that may alter testing dynamics. As we continue to combat COVID-19, integrating experience, expertise, authority, and trust into testing practices ensures that communities can navigate safely and confidently. The intersection of these elements fortifies our collective response, paving the way for better management of this and future pandemics.

Expertise from virologists and laboratory technicians has been invaluable in refining testing techniques. Enhanced methodologies, such as multiplex PCR that targets multiple sections of the virus’s genome, reduce false negatives and increase reliability. Collaborative efforts between medical institutions and biotech firms have accelerated technological advancements, ensuring rapid test development and efficient distribution. The role of authoritative bodies cannot be overstated. Institutions like the World Health Organization (WHO), the Centers for Disease Control and Prevention (CDC), and the Food and Drug Administration (FDA) provide stringent guidelines for test approval and use, safeguarding public health. Their ongoing research and data dissemination play a pivotal role in educating the public and health professionals, reinforcing trust in testing methodologies. Trustworthy information is the backbone of effective understanding and implementation of COVID-19 testing protocols. Reliable data from peer-reviewed studies and real-world applications offer insights into testing effectiveness and potential pitfalls. Regular updates from government and health authorities help dispel myths and encompass emerging variants that may alter testing dynamics. As we continue to combat COVID-19, integrating experience, expertise, authority, and trust into testing practices ensures that communities can navigate safely and confidently. The intersection of these elements fortifies our collective response, paving the way for better management of this and future pandemics.
Latest news
-
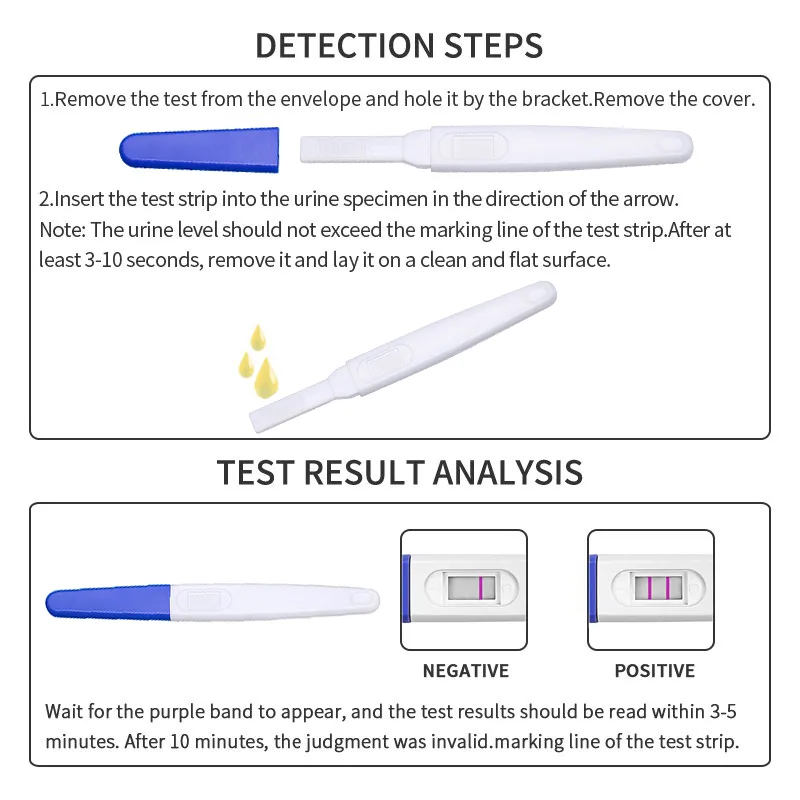
Highly Accurate hCG Pregnancy Test Strips - 5 Min Results
NewsAug.02,2025
-
Premium Empty ABS Plastic Cassettes: Durable & Lightweight Storage
NewsAug.01,2025
-
Accurate Cocaine (Coc) Rapid Test Kit | Fast & Reliable Detection
NewsJul.31,2025
-
Accurate HCG Pregnancy Test Strips | Fast Home Use Kit
NewsJul.31,2025
-
Reliable Early Pregnancy Test Kit Supplier - Multi Plastic Cassette Options
NewsJul.30,2025
-
Transferrin Rapid Test Cassette – Reliable Tumor Marker Detection
NewsJul.29,2025

