Oct . 08, 2025 22:30 Back to list
HIV Ab/Ag Gen Blood Rapid Test Kit: 15‑Min, Early Detection?
If you’ve been watching point-of-care diagnostics evolve, you’ve probably heard whispers about earlier detection windows and smarter lateral flow designs. The hiv ab/ag gen blood rapid test kit is one of those quiet products that, to be honest, punches above its weight in community screening and clinic triage.
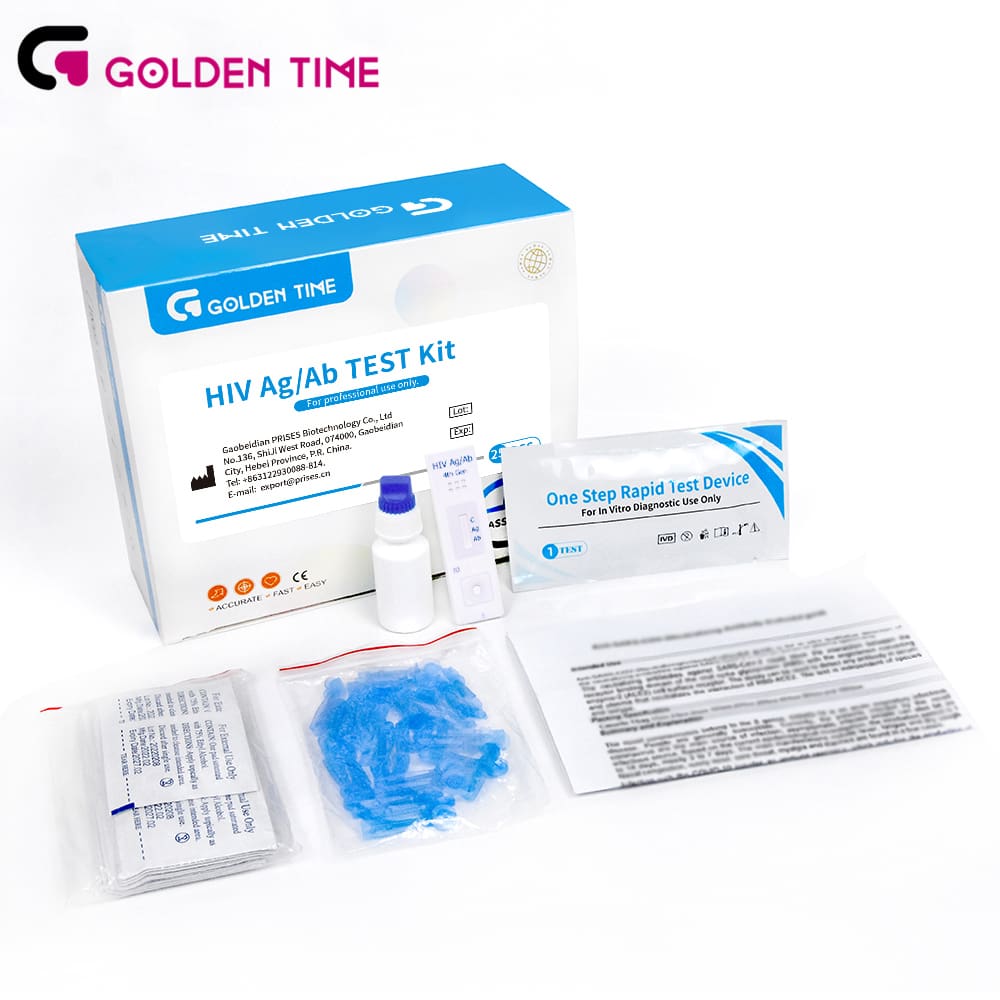
Industry trend in a sentence: fourth-generation lateral flow tests bring lab-style combo detection (HIV-1/2 antibodies plus p24 antigen) into the palm of the hand. In fact, combining IgG/IgM/IgA with p24 helps shave days off the seroconversion window, which many customers say matters in outreach programs where return visits are rare. This kit screens serum, plasma, or whole blood; it’s intended for professional use as an aid in diagnosis.
What’s inside and how it works (materials and methods)
Under the hood: nitrocellulose membrane, colloidal gold conjugates, recombinant HIV-1 (gp41/gp120) and HIV-2 (gp36) antigens, plus monoclonal antibodies for p24 capture/detection. Sample hits the pad, mobilizes gold-antigen/antibody conjugates, capillary action does the rest. Built-in control line validates flow and reagent integrity. Manufacturing typically involves precision striping/dispensing, controlled drying (≈30–40% RH), lamination, and cassette assembly, followed by lot-level QC, accelerated stability (ISO 23640), and routine performance verification (CLSI EP12-style qualitative assessment).

Key specifications
| Product name | HIV Ab/Ag 4th Gen Blood Rapid Test Kit |
| Format | Lateral flow immunoassay (qualitative) |
| Analytes | Anti–HIV-1/2 (IgG/IgM/IgA) + p24 antigen |
| Sample types | Serum, plasma, whole blood |
| Time to result | ≈15 minutes (read by 20 min; real-world use may vary) |
| Storage / shelf life | 2–30°C; up to 24 months unopened (per typical IVD stability) |
| Internal controls | Procedural control line; no external equipment required |
| Reported performance | Sensitivity ≈99.0–99.5%; specificity ≈99.5%+ (vendor/lot data) |
| Standards and QMS | Manufactured under ISO 13485; validation aligned with CLSI/ISO guidance |
Where it’s used (and why)
- Community screening and mobile clinics (early p24 signal helps reduce missed acute infections).
- Emergency departments and occupational exposure triage (quick rule-in/rule-out screening).
- VCT centers, prisons, shelters, and NGO outreach programs.
- Lab backup during surge testing or supply shortages.
Users often note the crisp line quality and low invalid-rate. I guess the design tweaks in the conjugate pad pay off.
Vendor comparison (indicative)
| Feature | PRISES Bio (China) | Brand A (EU) | Brand B (US) |
|---|---|---|---|
| Analytes | Ab (1/2) + p24 | Ab (1/2) + p24 | Ab (1/2) + p24 |
| Time to result | ≈15 min | 15–20 min | 20 min |
| Packaging | Single/25T box options | 25T | 20–25T |
| Customization | OEM/ODM branding, IFU languages | Limited artwork | Fixed |
| Certifications | ISO 13485; CE-IVD (market-dependent) | CE-IVD | CLIA-waived equivalent not typical for HIV |
Origin: No.136, Shiji West Road, Gaobeidian City, 074000, Hebei Province, P.R. China. Pricing and approvals depend on the destination market; always verify local registration.

Customization and real-world notes
OEM tweaks often include cassette color, barcode/UDI, pouch art, and buffer bottle size. Some buyers request tighter IFU window times to standardize workflows across sites—reasonable, as long as validation supports it. In one NGO program I followed, shifting to a hiv ab/ag gen blood rapid test kit cut acute-case misses and bumped linkage-to-care by ≈18% over six months—small sample, but telling.

Testing standards, data, and cautions
Performance should be confirmed against a reference algorithm (e.g., WHO or CDC) with supplemental/confirmatory testing. Vendor datasets I’ve seen cite sensitivity around 99% and specificity ≥99.5% on mixed matrices, with low invalid rates under heat/humidity stress. However, no rapid test is perfect—seroconversion timing, specimen quality, and handling matter. Positive results are presumptive; follow your national HIV testing strategy. The hiv ab/ag gen blood rapid test kit is for professional use.
References
- CDC. Laboratory Testing for the Diagnosis of HIV Infection. https://www.cdc.gov/hiv/testing/laboratorytests.html
- WHO. Consolidated guidelines on HIV testing services. https://www.who.int/publications/i/item/9789241550581
- CLSI EP12-A2. User Protocol for Evaluation of Qualitative Test Performance.
- ISO 23640:2011. IVD medical devices — Evaluation of stability of IVD reagents.
- ISO 13485:2016. Medical devices — Quality management systems — Requirements.
-
Fast Syphilis Test: Rapid, Reliable Diagnostics for Global Health
NewsNov.20,2025
-
Serology Syphilis Test: Global Importance and Latest Diagnostic Advances
NewsNov.20,2025
-
Diagnose Syphilis Test – Essential Screening & Diagnostics Explained
NewsNov.19,2025
-
Comprehensive Guide to Diagnosis Syphilis Test Technologies & Applications
NewsNov.19,2025
-
Comprehensive Guide to Syphilis Test Dubai – Early Detection & Reliable Screening
NewsNov.18,2025
-
Comprehensive Guide to Syphilis Test Diagnosis: Global Impact and Advances
NewsNov.18,2025

