Nov . 07, 2025 10:50 Back to list
H. Pylori Ab Combo Rapid Test | Fast, Accurate, Easy
Field Notes on H. Pylori Ab Combo Testing: Fast Answers, Real-World Use
If you work in primary care or community screening, you’ve probably asked the same question I do: how fast can we triage dyspepsia without overcomplicating the workflow? That’s where the One Step H. pylori antibody test—often called H. Pylori Ab Combo in catalogs—earns attention. It’s a rapid chromatographic immunoassay that detects antibodies to H. pylori in whole blood, serum, or plasma. No analyzers, very little training. To be honest, that simplicity is why distributors keep asking for it.

Industry snapshot
Antibody tests aren’t the new shiny thing—urea breath and stool antigen tests grab headlines now—but for outreach clinics, pharmacies (where permitted), and mobile programs, H. Pylori Ab Combo kits still move because they’re inexpensive, fast (≈10–15 minutes), and easy to stock. In fact, many buyers use them to triage and then confirm with antigen or breath testing if needed, which seems practical.

Key specifications (real-world use may vary)
| Principle | Lateral flow immunochromatography (qualitative) |
| Sample types | Whole blood / Serum / Plasma |
| Time to result | ≈10–15 minutes |
| Specimen volume | ≈10–20 µL |
| Storage / Shelf life | 2–30°C; shelf life ≈24 months (unopened) |
| Typical performance | Sensitivity ≈85–95%, Specificity ≈90–98% vs ELISA (literature ranges) |
| Controls | Built-in procedural control line |

How it’s made and validated (briefly)
- Materials: nitrocellulose membrane, colloidal gold conjugate coated with H. pylori antigens, sample pad, absorbent pad, plastic cassette.
- Method: apply sample + buffer; antibodies (if present) bind conjugate and migrate to form visible lines.
- Testing standards: evaluated per qualitative assay guidance such as CLSI EP12-A2; stability per ISO 23640; risk management under ISO 14971; QMS aligned with ISO 13485.
- Service life: unopened devices typically last ≈24 months at 2–30°C; avoid humidity and heat spikes.
- Industries served: primary care, GI clinics, NGO/mobile health, diagnostics distributors, OEM/house brands.
Note: serology detects exposure; it can’t confirm current active infection on its own. Many customers pair H. Pylori Ab Combo with stool antigen for active disease confirmation—sensible, honestly.

Vendor landscape (quick comparison)
| Vendor | Certifications | Lead time | Customization | Price band |
|---|---|---|---|---|
| Prisesbio H. Pylori Antibody Test Kit | ISO 13485; CE-IVD availability depends on region | ≈2–4 weeks | Private label, IFU languages, buffer format | $$ |
| Brand X Combo Ab | ISO 13485; CE-IVD | ≈3–6 weeks | Carton art, pouch color | $$$ |
| Budget Vendor Y | Basic QMS; regional approvals | ≈1–2 weeks | Limited | $ |
Origin (manufacturer address): No.136, Shiji West Road, Gaobeidian City, 074000, Hebei Province, P.R. China.
Use cases, feedback, and customization
- Walk-in clinics triaging dyspepsia within a single visit; confirm positives as per local policy.
- NGO screening in rural areas with limited power and cold chain.
- Distributors report that clear line intensity and simple IFU reduce operator errors—surprisingly impactful.
- OEM options: private label, multilingual IFUs, bundled lancets/buffers, and carton customization.
Mini case: A regional chain in Southeast Asia reported cutting average GI triage time from 45 minutes to ≈12 minutes by adopting H. Pylori Ab Combo at intake, then routing positives to stool antigen confirmation. Not perfect, but it smoothed patient flow.
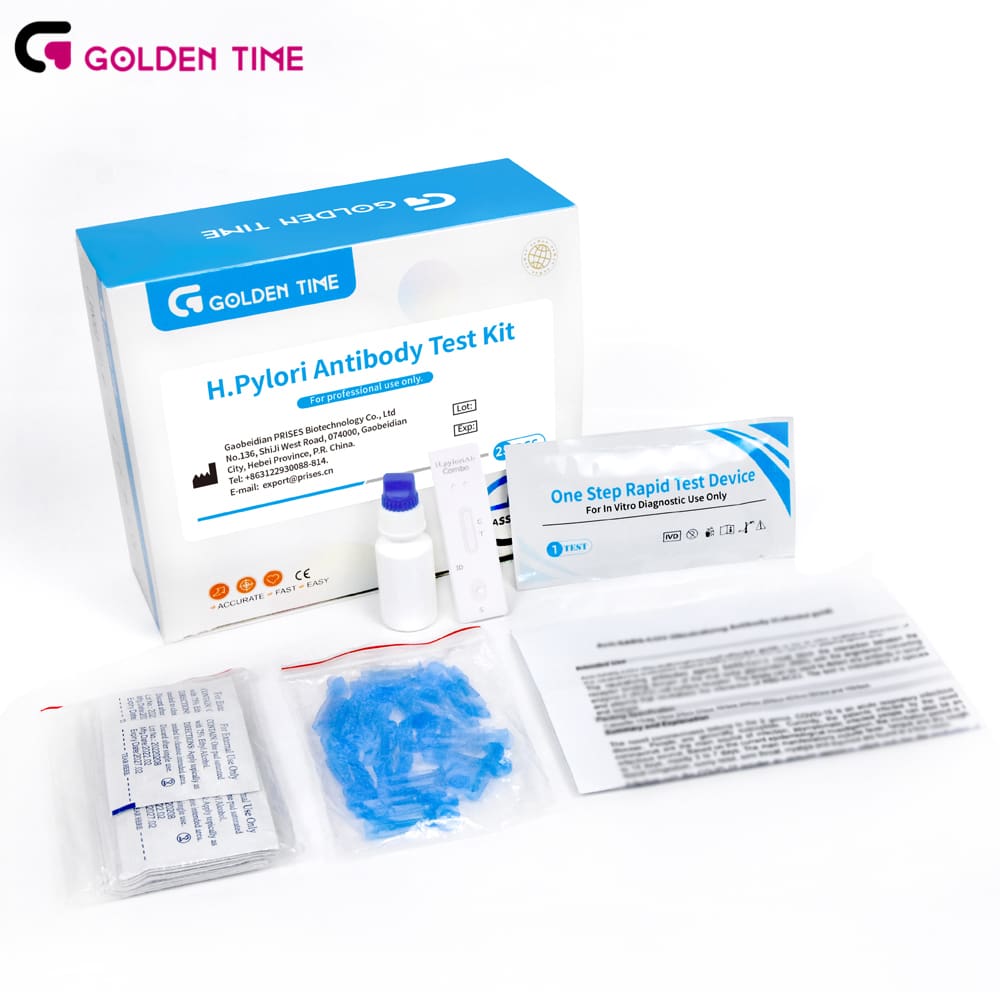
Practical notes and standards
Always follow local guidelines: many recommend antigen or breath tests for active infection. When you do use serology, anchor validation to CLSI EP12-A2, document stability (ISO 23640), and ensure supplier QMS (ISO 13485). I guess that sounds formal—but it saves headaches in audits.
- World Health Organization. Helicobacter pylori fact sheet.
- ACG Clinical Guideline: Treatment of Helicobacter pylori Infection (2022 update).
- CLSI EP12-A2: User Protocol for Evaluation of Qualitative Test Performance.
- ISO 23640: In vitro diagnostic medical devices — Evaluation of stability.
- ISO 13485: Medical devices — QMS requirements for regulatory purposes.
- EU Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR).
-
Fast Syphilis Test: Rapid, Reliable Diagnostics for Global Health
NewsNov.20,2025
-
Serology Syphilis Test: Global Importance and Latest Diagnostic Advances
NewsNov.20,2025
-
Diagnose Syphilis Test – Essential Screening & Diagnostics Explained
NewsNov.19,2025
-
Comprehensive Guide to Diagnosis Syphilis Test Technologies & Applications
NewsNov.19,2025
-
Comprehensive Guide to Syphilis Test Dubai – Early Detection & Reliable Screening
NewsNov.18,2025
-
Comprehensive Guide to Syphilis Test Diagnosis: Global Impact and Advances
NewsNov.18,2025

