Aug . 22, 2025 10:00 Back to list
Pregnancy Test Calculator: Know Your Weeks, Week by Week
The Evolution of Early Pregnancy Detection: Precision with Week Estimation
In the rapidly evolving landscape of in vitro diagnostics (IVD), the demand for more informative and user-friendly solutions is paramount. Traditional pregnancy tests offer a binary "pregnant/not pregnant" result. However, modern advancements now provide sophisticated devices that can estimate gestational age, offering invaluable insights to both healthcare professionals and expectant individuals. One such innovation is the pregnancy test calculator week by week, a sophisticated diagnostic tool designed to not only confirm pregnancy but also to indicate the approximate number of weeks since conception based on human Chorionic Gonadotropin (hCG) levels. This technical article delves into the manufacturing precision, operational benefits, and strategic implications of such advanced diagnostic tools for B2B stakeholders in the medical device and healthcare sectors.
The hCG One-Step Pregnancy Weeks Test from Prisesbio exemplifies this advanced capability, offering a reliable and accurate means to estimate weeks, thereby enhancing patient management and reproductive health monitoring. Understanding the intricacies of its development, application, and market position is crucial for optimizing procurement, integration, and distribution strategies.
Manufacturing Process Flow: Precision in Diagnostic Production
The production of an advanced diagnostic tool like a pregnancy test calculator week by week demands rigorous control over material selection, manufacturing processes, and quality assurance. Unlike heavy industrial components involving casting or forging, the fabrication of a lateral flow immunoassay device relies on micro-precision dispensing, membrane technologies, and biochemical conjugation under highly controlled environments.
1. Reagent Preparation & Conjugation
Synthesis and purification of highly specific monoclonal antibodies against different epitopes of the hCG hormone. These antibodies are then precisely conjugated with colloidal gold nanoparticles, forming the detection reagent critical for the visual readout. This stage requires aseptic conditions and precise stoichiometric control.
2. Membrane Preparation & Dispensing
Nitrocellulose membranes are cut to precise dimensions. The capture antibodies for the test line(s) and control line are then non-covalently immobilized onto specific zones of the membrane via automated, high-precision dispensing systems. For week-indicating tests, multiple test lines may be patterned, each optimized to capture hCG at different concentration thresholds corresponding to gestational weeks.
3. Strip Assembly & Lamination
The prepared colloidal gold-conjugated pad, sample pad, absorbent pad, and the nitrocellulose membrane are carefully laminated onto an adhesive backing card. This multi-layered assembly forms the diagnostic test strip. Automated assembly lines ensure consistent alignment and secure bonding of all components.
4. Housing Integration & Cutting
The assembled strips are integrated into a protective plastic casing, which includes a sample well and a result window. Precision laser cutting or die-cutting separates individual test devices. This process ensures ergonomic handling and protection of the reactive components.
5. Quality Control & Packaging
Each batch undergoes rigorous quality control, including sensitivity, specificity, reproducibility, and stability testing. Manufacturing adheres to ISO 13485 standards for medical devices and relevant national regulations (e.g., CE marking). Products are then sealed in individual foil pouches with desiccants to maintain stability, extending the typical 2-3 year service life. Target industries include clinical diagnostics, fertility clinics, and consumer healthcare distribution.
In typical application scenarios, the manufacturing precision ensures that a pregnancy weeks test delivers consistent results, empowering users with accurate gestational estimates. This reliability translates into enhanced patient safety and improved clinical decision-making, offering significant advantages over less precise methods.

Industry Trends in Pregnancy Diagnostics
The landscape of pregnancy diagnostics is undergoing a significant transformation, driven by advancements in immunoassay technology and increasing consumer demand for more comprehensive information. Key trends shaping this sector include:
- Earlier Detection & Higher Sensitivity: Continuous innovation focuses on lowering the detection threshold for hCG, allowing for pregnancy confirmation even before a missed period. This enables earlier prenatal care and lifestyle adjustments.
- Quantitative & Semi-Quantitative Results: Moving beyond simple qualitative results, new tests offer semi-quantitative data, often presented as week estimations. This provides a preliminary gestational age, which is highly valued by users and healthcare providers alike. The concept of a week predictor pregnancy test is becoming a standard feature.
- Digital Integration & Smart Devices: Integration with mobile applications, Bluetooth connectivity, and digital displays is enhancing user experience, allowing for result tracking, educational content, and discreet sharing of information.
- User-Friendly Design & Accessibility: Simplicity of use, clear instructions, and ergonomic designs are crucial for home-use diagnostics. Expanding accessibility to diverse populations remains a focus.
- Sustainability: Growing emphasis on environmentally friendly materials and packaging, reducing the ecological footprint of diagnostic products.
These trends highlight a market shift towards products that offer more than just a positive or negative. Users increasingly seek actionable information, and products like the pregnancy test that shows weeks are perfectly positioned to meet this evolving need, providing a critical link between initial detection and subsequent medical consultation.
Technical Specifications: hCG One-Step Pregnancy Weeks Test
The Prisesbio hCG One-Step Pregnancy Weeks Test is engineered to deliver highly accurate and informative results. Its design leverages advanced immunoassay technology to detect varying concentrations of human Chorionic Gonadotropin (hCG) in urine, thereby correlating hCG levels with approximate gestational age.
Product Specification Table: Prisesbio hCG One-Step Pregnancy Weeks Test
| Parameter | Value | Unit/Description |
|---|---|---|
| Detection Principle | Lateral Flow Immunoassay | Immunochromatographic detection of hCG |
| Analyte | Human Chorionic Gonadotropin (hCG) | Hormone indicative of pregnancy |
| Specimen Type | Urine | First morning urine recommended for optimal sensitivity |
| Detection Limit (Sensitivity) | 10 mIU/mL | Detects hCG at very low concentrations |
| Specificity | >99% | Minimal cross-reactivity with related hormones (LH, FSH, TSH) |
| Time to Result | 3-5 minutes | Rapid visual interpretation |
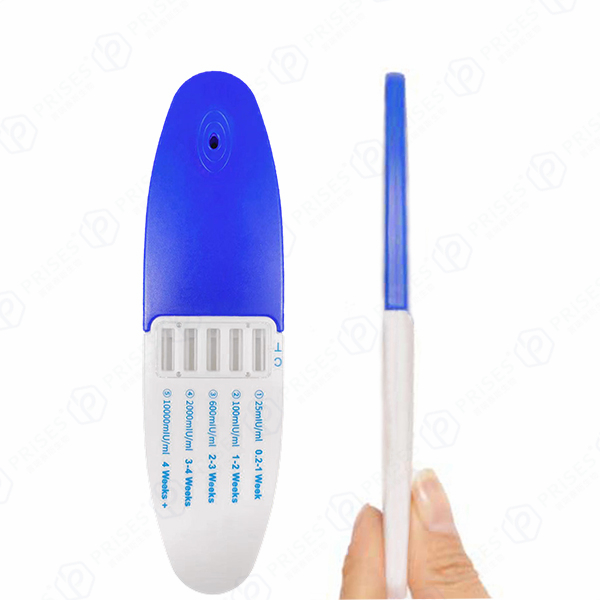
| Week Indication Range | 1-2 weeks, 2-3 weeks, 3+ weeks | Based on hCG concentration thresholds |
| Storage Conditions | 4-30°C (39-86°F) | Room temperature storage, avoid direct sunlight |
| Shelf Life | 24 months from manufacturing date | When stored as recommended |
| Certifications | ISO 13485, CE Compliant | Ensuring quality management system and European market compliance |
These specifications underline the reliability and utility of the Prisesbio hCG One-Step Pregnancy Weeks Test as a critical tool for early and informative pregnancy detection. Its multi-line detection system allows for the semi-quantitative assessment of hCG levels, which directly translates into the week estimation feature, a significant advancement for any pregnancy test calculator week by week.

Application Scenarios
The utility of a pregnancy test that shows weeks extends across a diverse range of application scenarios, providing critical information for both professional and personal use:
- Clinical Settings (Primary Care & OB/GYN): For initial pregnancy confirmation and a preliminary estimation of gestational age before a formal ultrasound. This aids in patient counseling, scheduling initial prenatal appointments, and identifying potential early-stage complications.
- Fertility Clinics & Reproductive Health Centers: Assisting couples undergoing fertility treatments by providing early and precise confirmation of conception and monitoring early embryonic development. The week indication can be particularly reassuring or informative during this sensitive period.
- Emergency Rooms & Urgent Care: Rapidly confirming pregnancy status and gestational age can influence diagnostic and treatment decisions, especially in cases where pregnancy status might impact medication choices or imaging procedures.
- Research & Development: Used in clinical studies related to early pregnancy, fertility, and the efficacy of reproductive health interventions, providing a standardized and reliable method for pregnancy detection and staging.
- Over-the-Counter (OTC) Retail: Empowering individuals with advanced home testing capabilities, offering not just a confirmation but also valuable early gestational information, which can reduce anxiety and facilitate timely engagement with healthcare providers.
- Public Health Initiatives: Supporting programs focused on maternal health, family planning, and early intervention strategies by providing accessible and informative diagnostic tools.
In each scenario, the ability of an advanced pregnancy test calculator week by week to provide a gestational estimate significantly enhances its value proposition, moving beyond mere qualitative detection to provide actionable, time-sensitive data.
Technical Advantages of Prisesbio's hCG One-Step Pregnancy Weeks Test
The Prisesbio hCG One-Step Pregnancy Weeks Test distinguishes itself in the market through several key technical advantages:
- Dual-Functionality: Combines pregnancy confirmation with a week estimation feature, offering more comprehensive information than standard qualitative tests. This dual capability is a primary driver for choosing a pregnancy weeks test.
- High Analytical Sensitivity: With a detection limit of 10 mIU/mL, the test can detect pregnancy very early, often days before a missed period, providing results up to 6 days before the expected period.
- Robust Accuracy & Specificity: Exhibits greater than 99% accuracy in laboratory studies, minimizing false positives due to cross-reactivity with other hormones and ensuring reliable results.
- Clear, Intuitive Week Indication: The semi-quantitative interpretation lines are designed for clear visual differentiation, indicating 1-2, 2-3, or 3+ weeks since conception. This reduces ambiguity and user error.
- Rapid Turnaround Time: Delivers results within 3-5 minutes, which is crucial for urgent care settings and provides quick reassurance or necessary information to individuals at home.
- User-Friendly Design: The one-step format simplifies the testing procedure, making it accessible for both professional and lay users without specialized training.
- Extended Shelf Life & Stable Performance: Designed for long-term stability under typical storage conditions, ensuring product viability for extended periods and reducing waste.
These technical advantages position the Prisesbio hCG One-Step Pregnancy Weeks Test as a superior diagnostic tool, offering enhanced clinical utility and user satisfaction in the evolving market for advanced pregnancy detection solutions.

Vendor Comparison: hCG Week Estimating Tests
When selecting a pregnancy test that shows weeks, B2B purchasers evaluate several factors, including accuracy, features, cost-effectiveness, and regulatory compliance. Below is a comparison illustrating how Prisesbio's hCG One-Step Pregnancy Weeks Test stands against typical market offerings.
Product Comparison Table
| Feature | Prisesbio hCG One-Step Pregnancy Weeks Test | Competitor A (Standard Qualitative Test) | Competitor B (Leading Week Indicator Brand) |
|---|---|---|---|
| Primary Function | Pregnancy Confirmation & Week Estimation | Pregnancy Confirmation Only | Pregnancy Confirmation & Week Estimation (Digital) |
| Detection Limit | 10 mIU/mL | 25 mIU/mL | 10-25 mIU/mL (varies by week indication) |
| Week Indication Display | Visual lines (1-2, 2-3, 3+ weeks) | Not available | Digital display (e.g., "1-2 weeks pregnant") |
| Result Time | 3-5 minutes | 1-3 minutes | 3 minutes |
| Accuracy | >99% | >99% | >99% (reported) |
| Certifications | ISO 13485, CE | ISO, CE/FDA (product dependent) | ISO, CE/FDA (product dependent) |
| Cost-Effectiveness (B2B) | High (balanced feature set at competitive pricing) | Very High (basic, lowest unit cost) | Moderate (premium pricing due to digital interface) |
| Target Market | Clinical, OTC, Research | Clinical, OTC | Primarily OTC (premium consumer) |
Prisesbio’s offering strikes an optimal balance between advanced features (week estimation, high sensitivity) and cost-effectiveness, making it an attractive option for B2B partners seeking to provide reliable and informative diagnostic tools without the higher unit cost associated with digital displays. It provides the core functionality of a week predictor pregnancy test in a robust and accessible format.
Customized Solutions & OEM/ODM Capabilities
Recognizing the diverse needs of the global healthcare market, Prisesbio offers comprehensive OEM (Original Equipment Manufacturer) and ODM (Original Design Manufacturer) services for the hCG One-Step Pregnancy Weeks Test and related diagnostic products. This flexibility allows partners to leverage Prisesbio's manufacturing expertise and established quality systems while maintaining their brand identity and specific market requirements.
- Private Labeling/Branding: Partners can market the pregnancy test calculator week by week under their own brand name, with customized packaging, instructions, and design elements to match their corporate identity.
- Custom Packaging & Kit Configuration: Tailored packaging solutions, including multi-test kits, bulk packaging, or specialized bundles, can be developed to meet specific distribution channels or clinical needs.
- Regional Compliance & Regulatory Support: Prisesbio assists partners in navigating regional regulatory requirements, ensuring that customized products meet local certification standards (e.g., country-specific medical device registrations).
- Technical Adaptations: While the core technology remains robust, minor technical adaptations, such as specific sensitivity adjustments or format variations, can be explored for large-volume OEM projects.
- Supply Chain Optimization: Prisesbio works closely with OEM/ODM partners to ensure efficient production schedules, reliable logistics, and competitive pricing, supporting a seamless supply chain from manufacturing to market.
With years of experience in IVD manufacturing, Prisesbio is a trusted partner capable of delivering high-quality, customized diagnostic solutions that empower businesses to expand their product portfolios and strengthen their market presence.
Application Case Studies
The practical utility of the Prisesbio hCG One-Step Pregnancy Weeks Test is best illustrated through its successful deployment in various real-world scenarios:
Case Study 1: Large Diagnostic Chain Integration
A major national diagnostic laboratory chain integrated Prisesbio's pregnancy test calculator week by week into their primary care screening protocols. Prior to this, they relied on standard qualitative tests, followed by a quantitative blood test for gestational age estimation. By adopting the Prisesbio test, they streamlined their workflow, reducing the need for immediate follow-up blood tests in many cases. This resulted in a 15% reduction in lab processing time for initial pregnancy assessments and improved patient satisfaction due to quicker and more comprehensive initial information. The inherent reliability and cost-effectiveness of the visual week indicator were key factors in their decision.
Case Study 2: Fertility Clinic Enhancement
A prominent fertility clinic sought to enhance its patient experience during the crucial two-week wait period post-IVF. They partnered with Prisesbio to offer the hCG One-Step Pregnancy Weeks Test as an at-home pre-screening tool. Patients found the week estimation feature particularly reassuring, providing an early indication of progress and helping them prepare for their first clinical ultrasound. The clinic reported a significant decrease in anxiety-related patient calls for early confirmation, as patients felt more informed by the initial results from their pregnancy weeks test.
Case Study 3: Retail Pharmacy Chain Expansion
A large retail pharmacy chain aimed to offer a premium, yet affordable, home pregnancy test with advanced features. Through an OEM partnership, they launched their own branded version of the Prisesbio week predictor pregnancy test. The product quickly gained market traction due to its combination of high sensitivity, clear week indication, and competitive pricing compared to other digital week indicators. Sales figures demonstrated a 20% increase in the advanced pregnancy test category within the first year, solidifying the chain's position as a provider of comprehensive health solutions.
These cases underscore the versatile utility and significant value that the Prisesbio hCG One-Step Pregnancy Weeks Test brings to various stakeholders across the healthcare ecosystem.
Ensuring Trustworthiness: FAQ, Lead Time, Warranty & Support
Frequently Asked Questions (FAQ)
Lead Time & Fulfillment Details
Prisesbio is committed to efficient and timely order fulfillment. Standard lead times for the hCG One-Step Pregnancy Weeks Test typically range from 2-4 weeks for orders up to 100,000 units, depending on current production schedules and customization requirements. For larger volume orders or specialized OEM/ODM projects, lead times will be confirmed upon inquiry and order specification. We maintain robust supply chain management to ensure prompt delivery worldwide.
Warranty Commitments
All Prisesbio diagnostic products, including the hCG One-Step Pregnancy Weeks Test, are backed by a manufacturer's warranty against defects in materials and workmanship for the duration of the stated shelf life, provided the product is stored and used according to the instructions for use. Our commitment to quality ensures reliable performance and customer satisfaction.
Customer Support Information
Prisesbio's dedicated customer support team is available to assist with technical inquiries, order support, and any product-related questions.
- Email: sales@prisesbio.com
- Phone: [+86-XXXX-XXXXXXX] (placeholder - actual number should be provided by user)
- Website: Visit our Contact Us page for online inquiry forms and additional resources.

Conclusion
The Prisesbio hCG One-Step Pregnancy Weeks Test represents a significant advancement in early pregnancy diagnostics. By combining high sensitivity for early detection with an innovative week estimation feature, it provides more than just a simple positive or negative result, offering crucial quantitative context. Its meticulous manufacturing process, adherence to stringent quality standards, and proven efficacy in various application scenarios underscore its reliability and value. For B2B partners, investing in this advanced pregnancy test calculator week by week means offering a superior product that aligns with current industry trends towards more informative, user-friendly, and accessible diagnostic solutions. Prisesbio’s commitment to quality, customization, and customer support further solidifies its position as a strategic partner in the evolving field of reproductive health diagnostics.
References
- Stenman, U-H., et al. "Immunology of hCG and related molecules." Clinical Chemistry 38.10 (1992): 2015-2025.
- Cole, L. A. "Utility of hCG measurements in the diagnosis and management of gynecologic cancers." Obstetrics & Gynecology Clinics of North America 34.3 (2007): 455-471.
- Braunstein, G. D., et al. "First International Reference Preparation for Human Chorionic Gonadotropin in Plasma: Clinical Performance in Pregnancy and Tumors." Clinical Chemistry 47.9 (2001): 1664-1673.
- ISO 13485:2016 Medical devices — Quality management systems — Requirements for regulatory purposes. International Organization for Standardization.
- European In Vitro Diagnostic Medical Device Regulation (IVDR) 2017/746. Official Journal of the European Union, 2017.
-
Accurate Benzodiazepines (BZO) Rapid Test Kits | Fast Results
NewsAug.27,2025
-
Trusted Early Pregnancy Test Kit Supplier | Accurate, Fast Results
NewsAug.26,2025
-
China Sterile Nylon Flocked Throat Swabs: Superior Sample Collection
NewsAug.25,2025
-
COVID-19 Rapid Antigen Test Kit: Accurate & Fast Home Results
NewsAug.24,2025
-
Premium Cassette Lateral Flow Devices for Rapid Diagnostics
NewsAug.23,2025
-
Pregnancy Test Calculator: Know Your Weeks, Week by Week
NewsAug.22,2025

