PRISES Biotechnology es un fabricante basado en I+D, dedicado al desarrollo, fabricación y comercialización de reactivos de diagnóstico (IVD) in vitro y equipos médicos, aprobado para la fabricación y comercialización de productos IVD de NMPA (CFDA) y operado bajo el sistema de calidad ISO 13485, la mayoría de los productos han sido certificados con la marca CE.
importante
productos
-

Uncut sheet
Uncut sheet
Hoja sin cortar para prueba rápida
-
Plastic Cassette
Plastic Cassette
Cassette de plástico para prueba rápida
-

Kit de prueba de enfermedades infecciosas
Kit de prueba de enfermedades infecciosas
Detects STDs, Hepatitis, H. pylori, Malaria, Dengue, Typhoid, Influenza and other diseases.
-

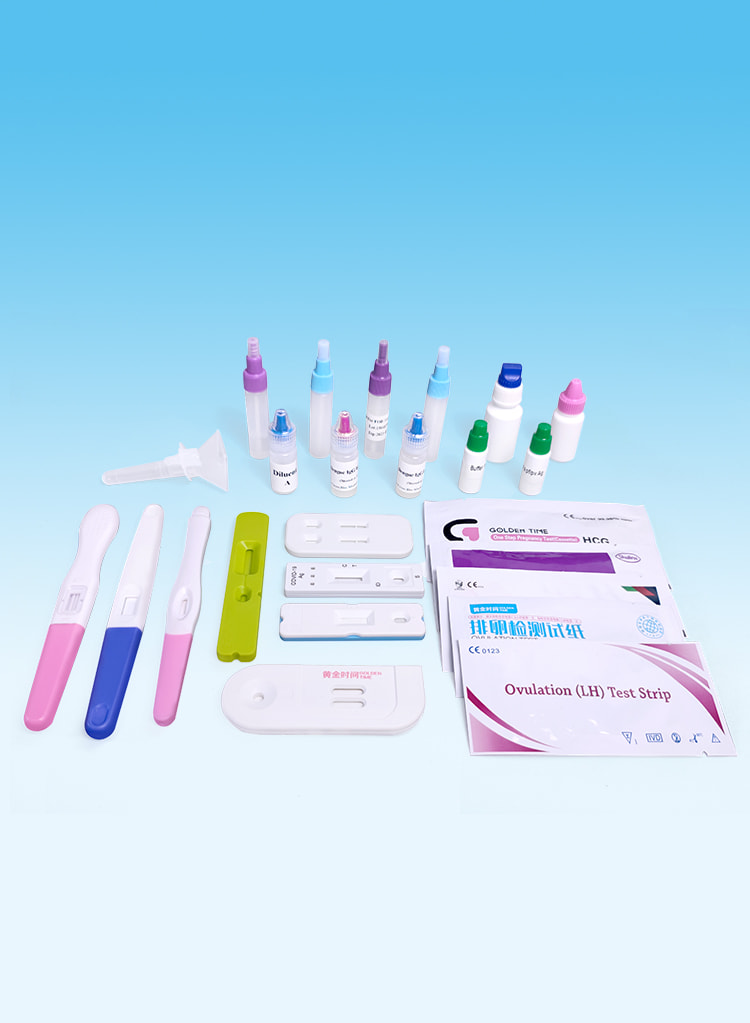
Pruebas de fertilidad
Pruebas de fertilidad
Effective detection of pregnancy and ovulation.
acerca de
PRISES
Our factory is founded in 2012 and located in Gaobeidian City, which is near Xiongan New Area and Beijing. It covers an area of 3,000 square meters, including class 1000,000 clean workshop with 1000 square meters, class 10 thousands of microbiological testing room with 500 square meters, well-equipped quality inspection rooms, research and development laboratories, etc.
-

10+
Business growth
10+
Business growthEstablished 11 years ago, the company has become a well-established and dynamic business through continuous efforts and improvements.
Ver detalles -

100000
Clean workshop
100000
Clean workshopClass 100,000 clean workshop 1000 square metres, Class 10,000 microbiological test room 500 square metres, fully equipped quality control room, R&D laboratory, laboratory, etc.
Ver detalles -

30+
Research staff
30+
Research staffThe company has a research and development team of 35 people who drive the technology and support the development of new products for the company.
Ver detalles -

20+
Consultant
20+
ConsultantWe provide the highest level of service to our customers, whether it's answering questions, solving problems or providing valuable advice, we deal with it promptly.
Ver detalles -

100+
Professionals
100+
ProfessionalsThe company has a large team of professionals responsible for managing, coordinating and controlling the operations of the various functions.
Ver detalles
noticias e informacion
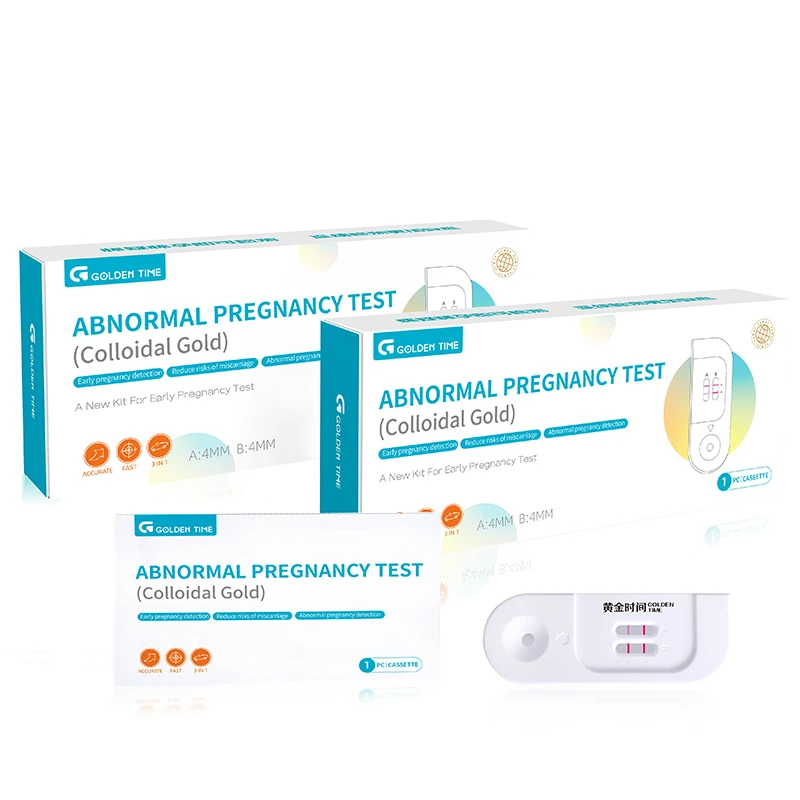
Understanding Pregnancy Tests: Exploring Key Options for Expecting Mothers
Pregnancy testing has evolved significantly in recent years, with more advanced and accurate tests available to expectant mothers.
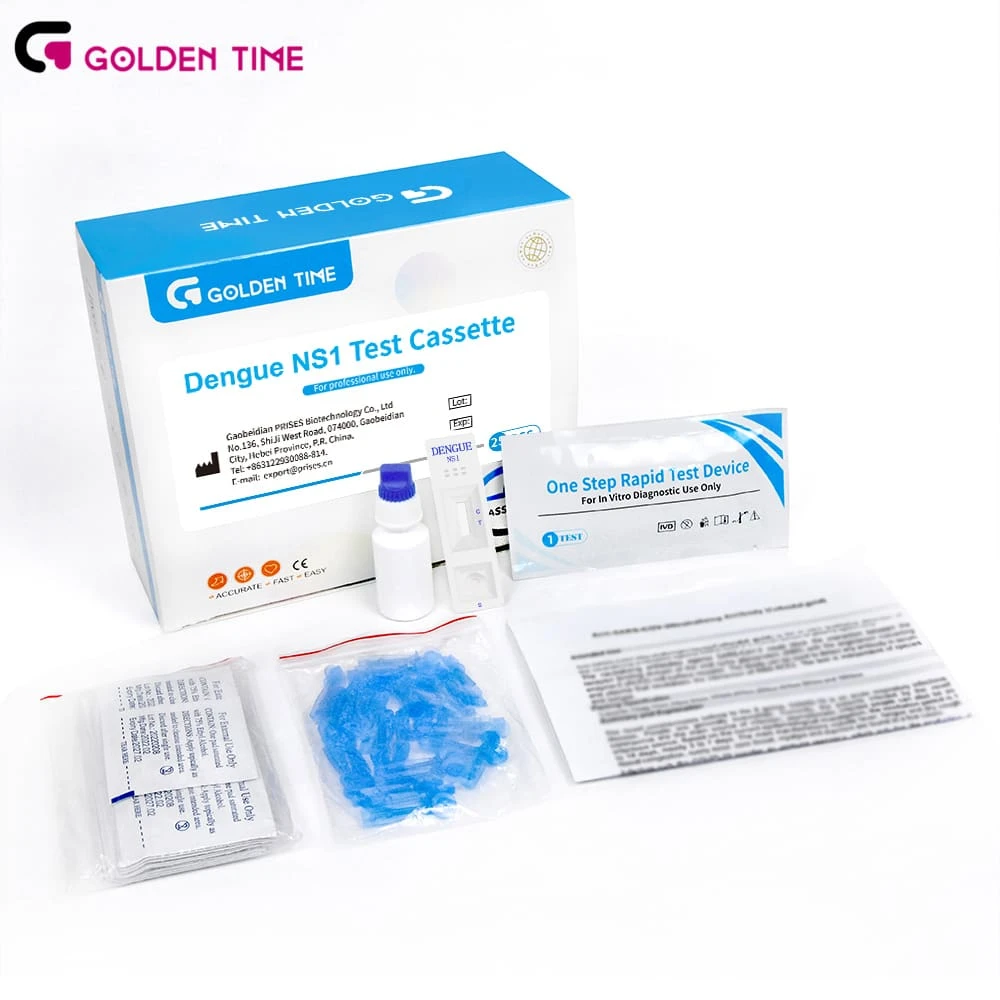
Quick and Reliable Dengue Testing Solutions
Dengue fever, caused by the dengue virus, is a rapidly spreading disease that can pose significant health risks.
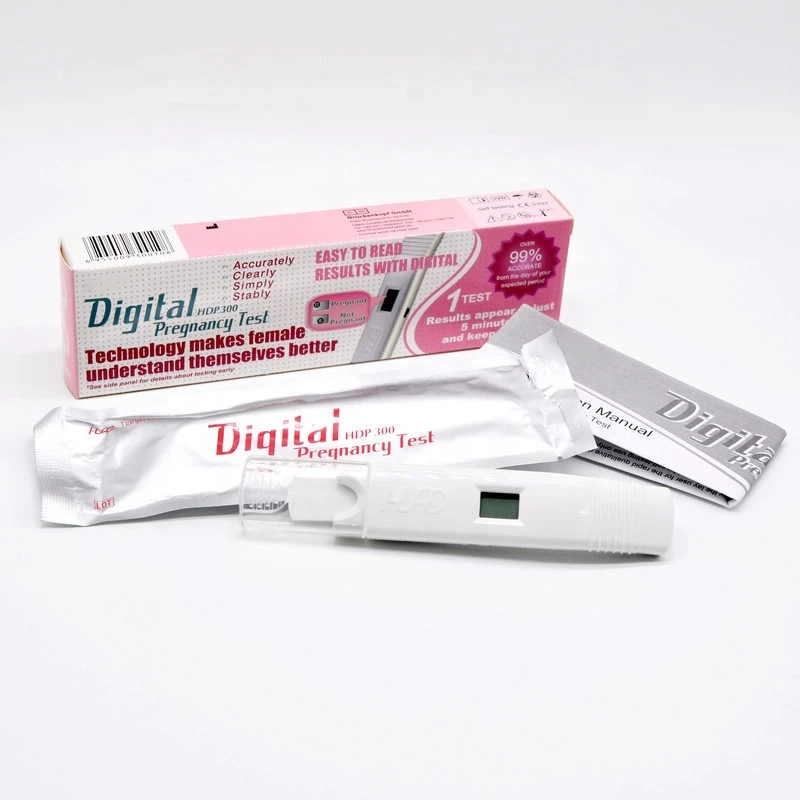
Early with Digital Pregnancy Tests
When you're eagerly awaiting news of a possible pregnancy, it's crucial to have accurate and reliable tests at your disposal.







