PRISES Biotechnology est un fabricant basé sur la R&D, engagé dans le développement, la fabrication et la commercialisation de réactifs de diagnostic in vitro (IVD) et d'équipements médicaux, qui a approuvé la fabrication et la commercialisation de produits IVD de NMPA (CFDA) et fonctionne sous le système de qualité ISO 13485, la plupart des produits ont été certifiés avec le marquage CE.
majeur
des produits
-

Uncut sheet
Uncut sheet
Feuille non découpée pour test rapide
-
Plastic Cassette
Plastic Cassette
Cassette en plastique pour test rapide
-

Kit de test de maladies infectieuses
Kit de test de maladies infectieuses
Detects STDs, Hepatitis, H. pylori, Malaria, Dengue, Typhoid, Influenza and other diseases.
-

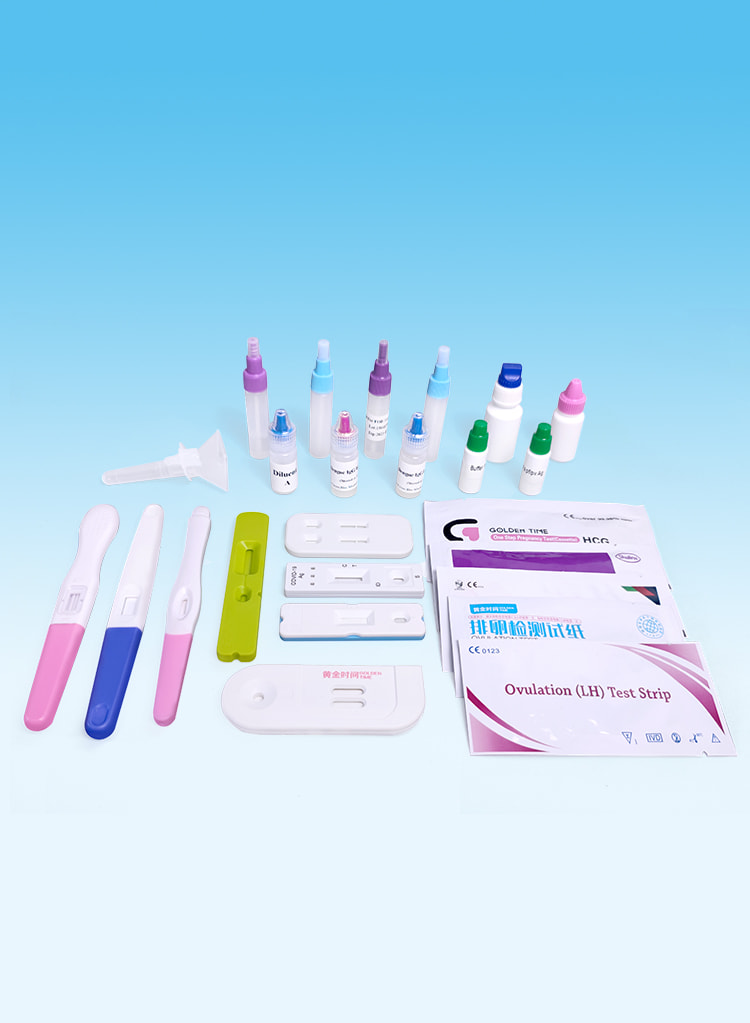
Tests de fertilité
Tests de fertilité
Effective detection of pregnancy and ovulation.
à propos
PRISES
Our factory is founded in 2012 and located in Gaobeidian City, which is near Xiongan New Area and Beijing. It covers an area of 3,000 square meters, including class 1000,000 clean workshop with 1000 square meters, class 10 thousands of microbiological testing room with 500 square meters, well-equipped quality inspection rooms, research and development laboratories, etc.
-

10+
Business growth
10+
Business growthEstablished 11 years ago, the company has become a well-established and dynamic business through continuous efforts and improvements.
Voir les détails -

100000
Clean workshop
100000
Clean workshopClass 100,000 clean workshop 1000 square metres, Class 10,000 microbiological test room 500 square metres, fully equipped quality control room, R&D laboratory, laboratory, etc.
Voir les détails -

30+
Research staff
30+
Research staffThe company has a research and development team of 35 people who drive the technology and support the development of new products for the company.
Voir les détails -

20+
Consultant
20+
ConsultantWe provide the highest level of service to our customers, whether it's answering questions, solving problems or providing valuable advice, we deal with it promptly.
Voir les détails -

100+
Professionals
100+
ProfessionalsThe company has a large team of professionals responsible for managing, coordinating and controlling the operations of the various functions.
Voir les détails
nouvelles et informations
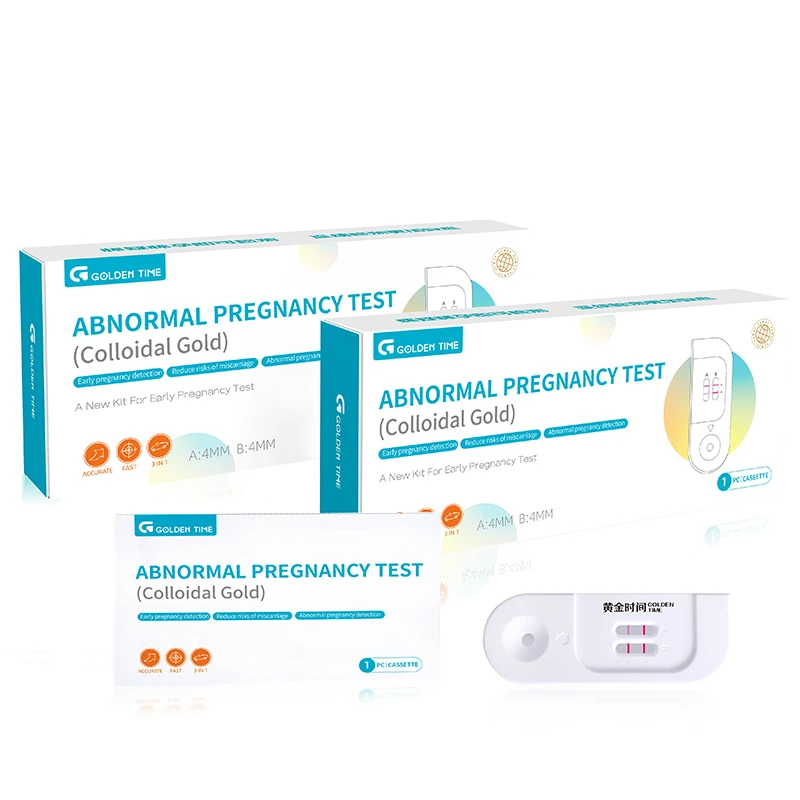
Understanding Pregnancy Tests: Exploring Key Options for Expecting Mothers
Pregnancy testing has evolved significantly in recent years, with more advanced and accurate tests available to expectant mothers.
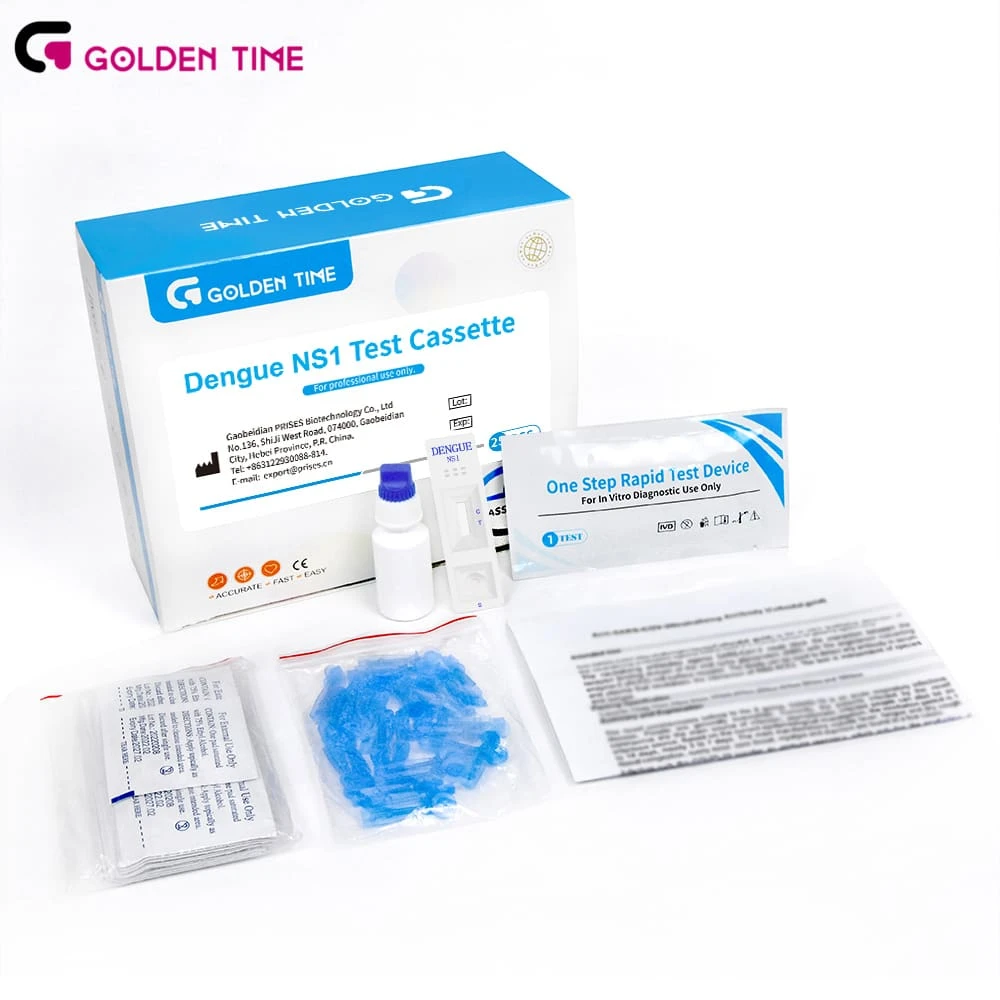
Quick and Reliable Dengue Testing Solutions
Dengue fever, caused by the dengue virus, is a rapidly spreading disease that can pose significant health risks.
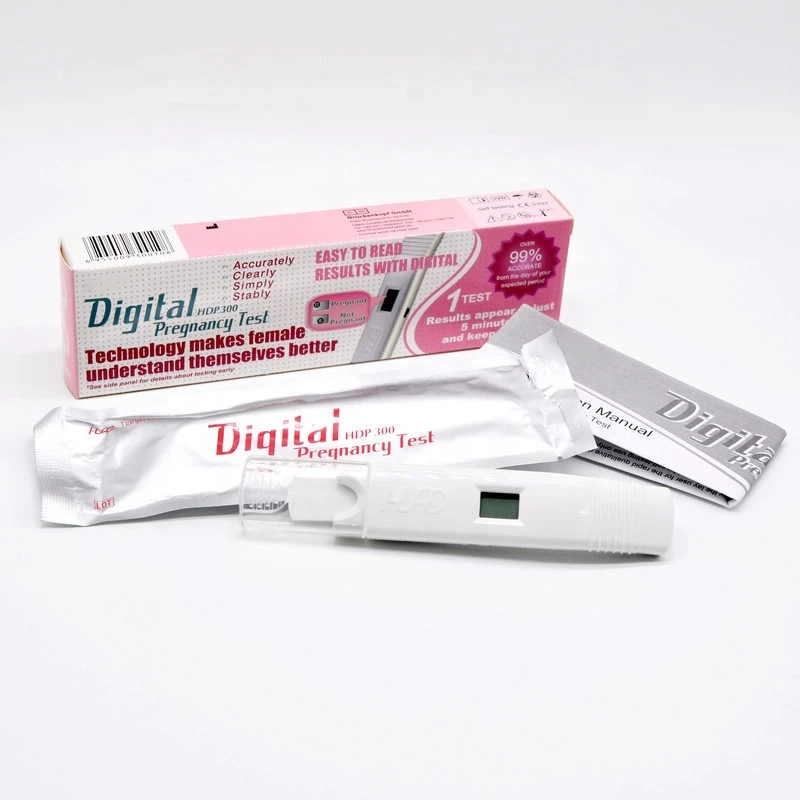
Early with Digital Pregnancy Tests
When you're eagerly awaiting news of a possible pregnancy, it's crucial to have accurate and reliable tests at your disposal.







