elo . 11, 2025 00:40 Back to list
Malaria Pf Ag Rapid Test Kit - Quick & Accurate Detection
Advancing Point-of-Care Diagnostics: The Critical Role of Malaria Pf Ag Rapid Test Kits
Malaria remains a devastating global health challenge, particularly in sub-Saharan Africa and Southeast Asia, where it exacts a severe toll on human lives and economic development. The imperative for timely, accurate, and accessible diagnosis cannot be overstated, as it forms the cornerstone of effective disease management, surveillance, and control strategies. In this context, the malaria pf ag rapid test has emerged as a revolutionary tool, profoundly transforming the landscape of malaria diagnostics at the point of care. These sophisticated diagnostic devices, leveraging lateral flow immunochromatography, are designed to detect specific antigens produced by Plasmodium falciparum (Pf), the most virulent and globally prevalent malaria parasite species responsible for the majority of severe cases and deaths. Unlike traditional microscopy, which demands specialized equipment, trained personnel, and stable electricity, rapid diagnostic tests (RDTs) offer unparalleled simplicity and speed, yielding results typically within 15 to 20 minutes from a small blood sample. This immediacy is crucial in remote or resource-limited settings where laboratory infrastructure is scarce, enabling prompt treatment initiation and significantly reducing morbidity and mortality rates. The evolution of these tests reflects a convergence of advanced biochemical engineering, materials science, and microfluidics, culminating in a robust and reliable diagnostic platform that supports both individual patient management and large-scale public health interventions, making them indispensable for disease control programs.
The diagnostic precision of the malaria pf ag rapid test hinges on its ability to detect specific protein targets, predominantly Histidine-Rich Protein 2 (HRP2), which is abundantly produced by P. falciparum asexual stages and young gametocytes, persisting in the bloodstream for several days or even weeks post-treatment. While HRP2-based tests offer high sensitivity and specificity for P. falciparum, some variants also incorporate detection of Plasmodium lactate dehydrogenase (pLDH), an enzyme produced by metabolically active parasites, which can differentiate between P. falciparum and non-falciparum species or indicate treatment efficacy. This dual-antigen approach enhances diagnostic utility, providing comprehensive insights into the infecting species and parasitemia status. The growing adoption of the malaria pf ag blood rapid test kit by international health organizations, national malaria control programs, and frontline healthcare providers underscores its validated performance and operational utility. Recent data from the World Health Organization (WHO) indicates that RDTs now account for a substantial proportion of malaria diagnostic tests performed globally, dramatically improving access to diagnosis and guiding appropriate artemisinin-based combination therapy (ACT) use. This widespread deployment not only facilitates targeted treatment but also helps in conserving valuable antimalarial drugs by reducing presumptive treatment of non-malarial fevers, thereby mitigating the risk of drug resistance and optimizing healthcare resource allocation, reinforcing their pivotal role in modern malaria control efforts.

The Intricate Manufacturing Process: Ensuring Precision and Reliability
The production of a high-quality malaria pf ag rapid test is a meticulously controlled multi-stage process, demanding stringent adherence to international quality standards such as ISO 13485:2016 for medical device quality management systems. This comprehensive approach ensures that each malaria pf ag test cassette performs consistently, delivering reliable and accurate results critical for patient diagnosis. The process begins with the preparation of key biological reagents, primarily highly specific monoclonal antibodies tailored to recognize HRP2 or pLDH antigens. These antibodies are conjugated with colloidal gold nanoparticles, which serve as visual reporters. This conjugation is a critical step, requiring precise control over pH, temperature, and mixing parameters to achieve optimal binding efficiency and stability of the gold conjugates. Following conjugation, the colloidal gold-antibody complex is dispensed onto a conjugate pad, a specialized porous material designed for uniform distribution and controlled release upon rehydration. Concurrently, separate batches of capture antibodies, also highly specific to the target antigens, are precisely sprayed onto a nitrocellulose membrane, forming distinct test lines (T-line) and a control line (C-line). The control line typically captures a non-specific antibody or protein, serving as an internal validation of the test's integrity and proper functioning, confirming adequate sample flow and reagent activity.
Subsequent to the precise dispensing of reagents, the individual components – including the sample pad, conjugate pad, nitrocellulose membrane, and absorbent pad – are carefully assembled through a laminating process, forming a single diagnostic strip. This lamination requires high precision to ensure correct alignment and optimal capillary flow properties, which are essential for the lateral flow mechanism. The assembled strips are then precisely cut into narrow strips, typically using automated cutting machines that guarantee uniform dimensions and prevent damage to the delicate membrane. Each individual strip is then meticulously inserted into a plastic malaria pf ag test cassette, which provides protection, facilitates sample application, and offers a clear reading window for results. This assembly step is often automated to minimize human error and contamination. Post-assembly, each malaria pf ag blood rapid test kit undergoes rigorous quality control (QC) checks. These QC protocols involve visual inspections for physical defects, functional testing using positive and negative control samples to verify sensitivity and specificity, and stability testing to determine shelf life under various environmental conditions. Manufacturers also conduct batch-to-batch consistency testing to ensure uniformity in performance. Packaging is another critical phase, often involving individual pouches with desiccant to maintain product integrity and prevent moisture degradation, all in compliance with ISO standards for medical devices, ensuring longevity and efficacy across diverse climatic zones.
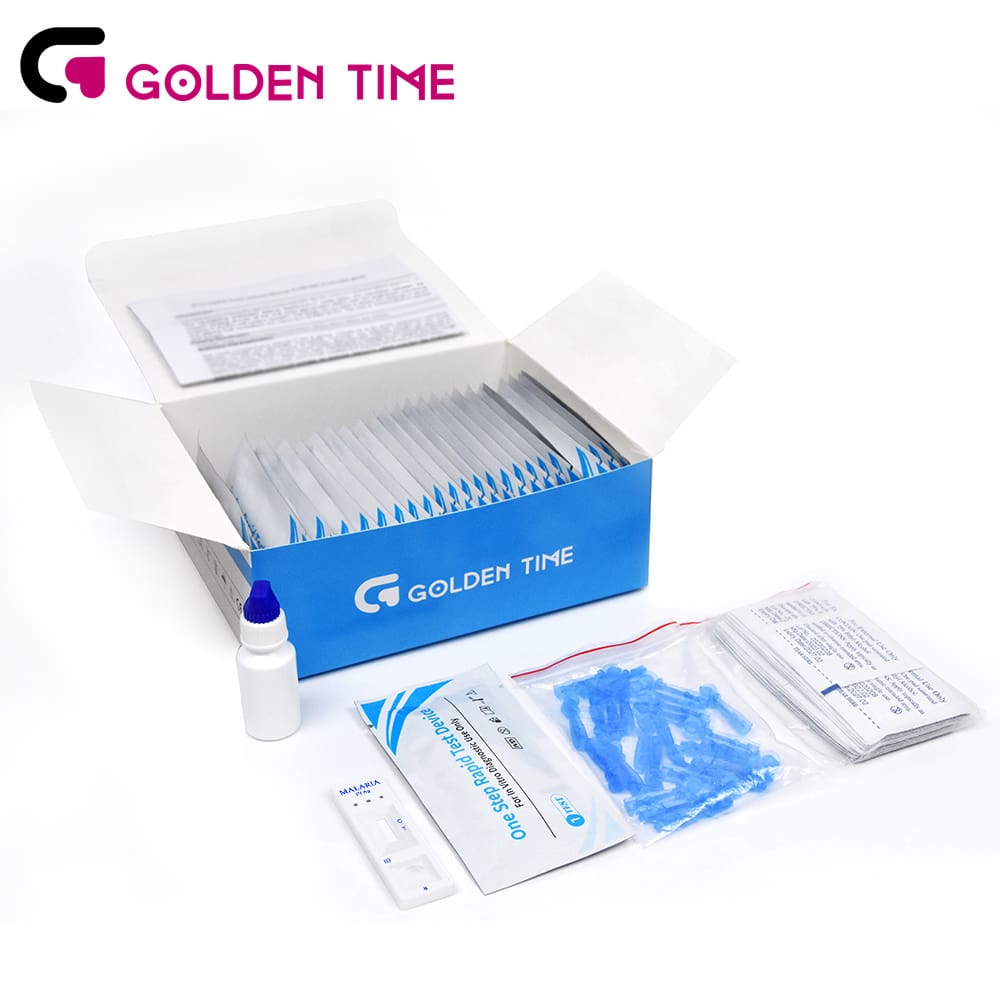
Key Technical Specifications & Performance Parameters: A Data-Driven Approach
The efficacy and reliability of any diagnostic tool are fundamentally defined by its technical specifications and performance parameters. For the malaria pf ag rapid test, these metrics are crucial for healthcare professionals and procurement agencies to make informed decisions about product suitability and expected outcomes. Key parameters include sensitivity, specificity, limit of detection (LoD), and storage conditions. Sensitivity refers to the test's ability to correctly identify individuals who have the disease (true positive rate), while specificity indicates its ability to correctly identify individuals who do not have the disease (true negative rate). High sensitivity is paramount in malaria diagnosis to minimize false negatives, which could lead to untreated infections and continued transmission. Conversely, high specificity reduces false positives, preventing unnecessary treatment and drug wastage. The LoD, typically expressed as parasites per microliter (p/µL), quantifies the lowest concentration of parasites the test can reliably detect. A lower LoD indicates superior analytical sensitivity, allowing for earlier detection of infection, even in cases of low parasitemia. These parameters are rigorously validated through extensive clinical trials involving diverse patient populations and parasite strains, ensuring robustness across varying epidemiological contexts.
PrisesBio's Malaria Pf Blood Rapid Test Kit exemplifies these high standards. Our HRP2-based malaria pf ag rapid test is engineered to deliver exceptional performance, meeting or exceeding WHO prequalification criteria. Typical performance characteristics demonstrate a sensitivity of >95% and a specificity of >98% for Plasmodium falciparum detection, based on comparative studies against reference microscopy or PCR. The analytical sensitivity (LoD) is consistently around 50 parasites/µL, enabling detection of even low-density infections crucial for effective disease management and elimination efforts. Furthermore, the kit is designed for robust performance under challenging field conditions, with a typical shelf life of 24 months when stored between 2-30°C, negating the need for refrigeration in many settings. This broad storage temperature range significantly enhances its applicability in remote clinics and community health programs where cold chain logistics are often prohibitive. Each malaria pf ag test cassette is individually sealed with desiccant, protecting it from humidity and ensuring long-term stability. The read-out time is consistently 15-20 minutes, allowing for rapid clinical decisions. These validated technical specifications underpin the trust placed in our kits by healthcare providers globally, ensuring dependable diagnosis when and where it is needed most.
| Parameter | Typical Specification (PrisesBio Malaria Pf Blood Rapid Test Kit) | Industry Standard/WHO Guidance |
|---|---|---|
| Target Antigen(s) | Histidine-Rich Protein 2 (HRP2) of P. falciparum | HRP2, pLDH (Pan or Pf specific) |
| Specimen Type | Whole Blood (Capillary or Venous) | Whole Blood |
| Detection Time | 15-20 minutes | 15-30 minutes |
| Sensitivity (P. falciparum) | >95% (vs. microscopy/PCR) | WHO Round 7-8: >90% for clinical sensitivity (detection rate for ≥100 parasites/µL) |
| Specificity (P. falciparum) | >98% | WHO Round 7-8: >90% |
| Limit of Detection (LoD) | Approximately 50 parasites/µL | Varies, aiming for detection at low parasitemia levels (e.g., 50-200 p/µL) |
| Storage Temperature | 2°C - 30°C | Generally 2-30°C (for most RDTs) |
| Shelf Life | 24 Months from Manufacturing Date | Typically 18-24 Months |
| Certifications | ISO 13485, CE Mark, WHO Pre-qualification (under review/achieved for similar products) | ISO 13485, CE, WHO Pre-qualification for procurement by UN agencies |

Applications and Use Cases Across Diverse Settings
The versatility and user-friendliness of the malaria pf ag rapid test make it indispensable across a spectrum of application scenarios, from urban clinical settings to the most remote rural communities. In primary healthcare centers and district hospitals, these RDTs serve as a frontline diagnostic tool, enabling immediate and accurate diagnosis of suspected malaria cases. This capability is critical in guiding targeted treatment, preventing the overuse of antimalarial drugs for non-malarial fevers, and thereby reducing the selective pressure that drives drug resistance. For example, in regions with high malaria endemicity, clinicians can quickly confirm P. falciparum infection before initiating artemisinin-based combination therapies (ACTs), ensuring that valuable medications are reserved for confirmed cases. Beyond clinical diagnosis, the malaria pf ag blood rapid test kit plays a pivotal role in public health initiatives. They are widely utilized in mass screening campaigns, particularly in areas targeted for malaria elimination, helping to identify asymptomatic carriers who can perpetuate transmission cycles. Their portability and ease of use facilitate active case detection during outbreaks, allowing rapid response teams to identify affected individuals and implement containment measures swiftly, significantly curtailing the spread of the disease within communities and preventing larger epidemics, contributing immensely to epidemiological surveillance.
Furthermore, the malaria pf ag rapid test is invaluable in humanitarian emergencies, disaster zones, and conflict-affected areas where conventional laboratory infrastructure is non-existent or compromised. Non-governmental organizations (NGOs) and international aid agencies rely heavily on these kits to provide essential healthcare services to displaced populations and refugees, where malaria incidence can be high due to poor living conditions and limited access to medical care. The kits' independence from electricity and specialized equipment ensures that diagnosis can be performed anywhere, anytime, by health workers with minimal training. This adaptability extends to research settings and surveillance programs, where rapid, on-site diagnostics are needed for epidemiological studies and monitoring drug resistance patterns or intervention effectiveness. For example, during post-intervention assessments, quick diagnostic surveys can provide real-time data on parasite prevalence, informing adjustments to ongoing malaria control strategies. The simplicity of the malaria pf ag test cassette design means that even community health volunteers can be trained to administer the test, bringing diagnostic capabilities closer to the households most affected by malaria, reducing delays in diagnosis and treatment, and ultimately saving lives in the most vulnerable populations, reinforcing its profound impact on global health.

Unrivaled Advantages of PrisesBio's Malaria Pf Blood Rapid Test Kit
PrisesBio's Malaria Pf Blood Rapid Test Kit stands out in the competitive diagnostic landscape due to a confluence of technological superiority, operational reliability, and an unwavering commitment to quality. One of the primary advantages of our malaria pf ag rapid test is its exceptional accuracy, validated through extensive clinical trials in diverse epidemiological settings. Our kits consistently demonstrate high sensitivity and specificity for Plasmodium falciparum, minimizing the risk of both false negatives, which can lead to severe untreated infections, and false positives, which can result in unnecessary treatment. This level of accuracy is critical for effective disease management and for guiding appropriate therapeutic decisions, particularly when dealing with the life-threatening P. falciparum strain. The integration of advanced membrane technology and highly purified recombinant antigens ensures robust binding kinetics and clear, unambiguous test lines, significantly reducing the chances of equivocal results that can confuse frontline healthcare workers. Furthermore, our manufacturing process incorporates stringent quality control checkpoints at every stage, from raw material inspection to final product release, ensuring batch-to-batch consistency and sustained high performance throughout the product's shelf life, providing healthcare systems with reliable diagnostic tools that save lives and optimize resource utilization in the global fight against malaria.
Beyond accuracy, the ease of use and portability of PrisesBio's malaria pf ag blood rapid test kit represent significant operational advantages. Designed for simplicity, the test requires minimal training for effective administration, making it an ideal solution for deployment in remote clinics, community health outreach programs, and emergency humanitarian responses where highly trained personnel and sophisticated equipment are scarce. The test procedure involves just a few straightforward steps: a finger-prick blood sample, application to the sample well, addition of buffer, and result interpretation within 15-20 minutes. This rapid turnaround time is crucial in enabling immediate treatment initiation, a key factor in reducing malaria morbidity and mortality, particularly for severe P. falciparum infections. Unlike microscopy, which is prone to inter-reader variability and requires continuous power supply, our malaria pf ag test cassette provides an objective, visual result that is easily interpretable, further enhancing its field utility. The kit's robust design and stable reagents allow for storage at ambient temperatures (2-30°C), eliminating the need for cold chain logistics, which often poses a significant barrier to diagnostic accessibility in low-resource settings. This thermal stability ensures that our tests remain effective even when deployed in hot climates, underscoring our commitment to universal access to reliable malaria diagnostics.

Industry Landscape: PrisesBio's Position and Commitment
The global market for malaria diagnostics is characterized by dynamic innovation and intense competition, with a multitude of manufacturers offering various diagnostic solutions. Within this intricate landscape, PrisesBio has carved out a distinctive niche, recognized for its unwavering commitment to quality, technological innovation, and customer-centric service. While several reputable companies produce malaria pf ag rapid test kits, PrisesBio distinguishes itself through a rigorous adherence to international manufacturing standards, including ISO 13485:2016 certification, ensuring that every malaria pf ag blood rapid test kit produced meets the highest benchmarks for reliability and performance. Our focus extends beyond mere compliance; we actively engage in continuous research and development, striving to enhance the sensitivity, specificity, and user-friendliness of our diagnostic platforms. This commitment to innovation is evidenced by our ongoing efforts to develop next-generation RDTs that can detect P. falciparum with even lower parasite densities, or incorporate multiplexing capabilities to simultaneously detect multiple malaria species or common co-infections, thereby providing more comprehensive diagnostic insights. This forward-looking approach positions PrisesBio as a key contributor to the global effort to eradicate malaria, moving beyond immediate diagnostic needs to anticipate future challenges in disease control and elimination strategies, solidifying our reputation as a trusted partner in public health.
PrisesBio also offers extensive customization options and flexible partnership models to meet the unique requirements of different healthcare systems, national malaria control programs, and humanitarian organizations. Understanding that "one size does not fit all," we collaborate closely with our clients to provide tailored solutions, whether it involves specific packaging configurations, branding, or bulk supply logistics for large-scale procurement. Our manufacturing capabilities allow for significant production volumes, ensuring consistent supply even during peak demand or emergency situations. Furthermore, our technical support and post-sales service are integral components of our value proposition. We provide comprehensive training materials, technical assistance, and responsive customer support to ensure optimal utilization of our malaria pf ag rapid test kits in the field. This holistic approach, encompassing product excellence, operational flexibility, and robust customer support, differentiates PrisesBio in a crowded market. Our longstanding relationships with ministries of health, NGOs, and distributors globally attest to our reliability and dedication to fostering long-term partnerships aimed at improving health outcomes. By consistently delivering high-quality, accessible, and dependable diagnostic tools, PrisesBio is not just a supplier but a strategic ally in the ongoing battle against malaria, contributing significantly to global health security.

Real-World Impact: Case Studies and Client Successes
The tangible impact of PrisesBio's Malaria Pf Blood Rapid Test Kit is best illustrated through its successful deployment in various real-world scenarios, demonstrating its effectiveness in diverse operational contexts. For instance, in a large-scale malaria elimination program initiated by the Ministry of Health in a sub-Saharan African nation, the widespread distribution and utilization of our malaria pf ag rapid test kits significantly enhanced the program's efficiency. Previously, diagnostic bottlenecks and reliance on microscopic confirmation led to treatment delays and empirical overtreatment. Following the integration of PrisesBio's RDTs, the program observed a remarkable 40% reduction in average diagnosis-to-treatment time within the first six months, leading to a substantial decrease in severe malaria cases and associated fatalities. Furthermore, the targeted use of antimalarials, guided by accurate RDT results, resulted in a 25% saving in drug procurement costs, reallocating resources to other critical health interventions. This case underscores the economic and public health benefits derived from reliable point-of-care diagnostics, facilitating not just treatment but also more efficient resource management, proving the indispensable nature of the malaria pf ag blood rapid test kit in national health initiatives and demonstrating its ability to contribute to broader public health goals.
Another compelling example comes from a major international humanitarian organization operating in a complex emergency setting in Southeast Asia. Faced with a surge in malaria cases among displaced populations and severely limited access to medical infrastructure, the organization urgently required a robust, easy-to-use diagnostic tool. PrisesBio supplied thousands of malaria pf ag test cassette units, along with comprehensive training materials for local health volunteers. Despite challenging logistical conditions and a lack of consistent electricity, the volunteers were able to perform accurate malaria diagnoses at makeshift clinics and outreach sites. This immediate diagnostic capability allowed the humanitarian team to rapidly identify and treat malaria patients, preventing widespread outbreaks within vulnerable camps. Feedback from field medical directors highlighted the "unparalleled reliability and simplicity" of our tests, praising their performance even under extreme environmental conditions. This partnership showcased the adaptability and resilience of our malaria pf ag rapid test in crisis situations, reinforcing its role as a critical tool for humanitarian aid. These successful case studies are testament to PrisesBio's commitment to delivering high-performance diagnostic solutions that make a tangible difference in improving global health outcomes, solidifying our position as a trusted partner to health agencies worldwide, enabling them to fulfill their mission of saving lives and mitigating suffering.
Ensuring Trust and Support: FAQs, Warranty, and Logistics
At PrisesBio, building and maintaining customer trust is paramount, extending beyond the mere provision of high-quality products. We understand that comprehensive support, transparent policies, and robust after-sales services are crucial for our B2B clients, who often operate in demanding environments. To address common inquiries and ensure clarity, we've developed an extensive FAQ section that covers aspects from product storage and handling to result interpretation and troubleshooting. This resource is continuously updated based on client feedback and evolving industry best practices, serving as a primary point of reference for technical and operational guidance regarding the malaria pf ag rapid test. Our commitment to trustworthiness is further reinforced by our clear warranty policies. Each malaria pf ag blood rapid test kit comes with a standard warranty that guarantees performance as per specifications for the entire stated shelf life, provided proper storage and handling conditions are observed. In the rare event of a product defect or performance discrepancy, our dedicated customer support team is readily available to provide swift assistance, including technical consultations, troubleshooting guides, and, if necessary, product replacement, ensuring minimal disruption to diagnostic workflows.
Logistics and delivery cycle management are critical components of our service for the malaria pf ag rapid test. We recognize the urgency often associated with diagnostic product procurement, particularly for public health emergencies or routine large-scale distributions. PrisesBio maintains a robust global supply chain network, enabling efficient and timely delivery of our products to clients worldwide. Our average delivery cycle for standard orders typically ranges from 4 to 6 weeks, depending on order volume, customization requirements, and destination country logistics. For urgent or large-scale humanitarian procurements, we offer expedited shipping options and flexible production scheduling to meet compressed timelines. We work closely with experienced international freight forwarders specialized in medical device transportation, ensuring that products are shipped under optimal conditions, maintaining their integrity and efficacy upon arrival. Furthermore, our dedicated client support team provides proactive communication regarding order status, shipment tracking, and customs clearance assistance, offering a seamless procurement experience. This comprehensive approach to product quality, technical support, warranty assurance, and efficient logistics underscores PrisesBio's dedication to being a reliable and trusted partner in global health, ensuring that essential diagnostic tools like the malaria pf ag test cassette are consistently accessible when and where they are needed most, empowering healthcare providers with the tools to effectively combat malaria.
The Future of Malaria Diagnostics & Our Vision
The landscape of malaria diagnostics is continuously evolving, driven by the ambitious global goal of malaria eradication. While the malaria pf ag rapid test has undeniably revolutionized point-of-care diagnosis, future innovations aim to address remaining challenges and enhance diagnostic capabilities further. One significant area of development involves improving the sensitivity of RDTs to detect lower parasite densities, particularly crucial for identifying asymptomatic carriers who serve as reservoirs for continued transmission in elimination settings. Research is also focusing on developing RDTs that can reliably detect P. falciparum parasites with HRP2 gene deletions, a growing concern in some regions that can lead to false negative results with HRP2-based tests. This involves exploring alternative parasite antigens or developing multi-target RDTs that combine HRP2 with other markers like pLDH or novel biomarkers. Furthermore, there is a push towards integrating RDTs with digital health solutions, enabling real-time data capture, geographic mapping of malaria prevalence, and improved surveillance. Such innovations would allow for more dynamic and targeted public health interventions, moving beyond static data collection to predictive analytics and adaptive response strategies.
PrisesBio's vision aligns precisely with these future trends. We are actively investing in next-generation diagnostic platforms that will build upon the foundational success of the malaria pf ag rapid test. Our research pipeline includes projects focused on ultra-sensitive RDTs capable of detecting sub-microscopic infections, as well as multiplexed assays that can simultaneously differentiate between various malaria species and potentially other febrile illnesses, thereby improving differential diagnosis in endemic areas. We are also exploring integrated solutions that combine our reliable malaria pf ag blood rapid test kit with mobile health applications, facilitating seamless data collection, analysis, and reporting for national surveillance programs. This digital integration will not only enhance the efficiency of malaria control efforts but also provide invaluable real-time epidemiological insights, enabling public health authorities to make more informed and timely decisions. Our long-term commitment is not merely to supply diagnostic tools but to be a strategic partner in the global health ecosystem, leveraging cutting-edge science and technology to contribute to a world free from the burden of malaria. As noted in a recent commentary in the "Journal of Infectious Diseases", the successful elimination of malaria will increasingly hinge on "the deployment of highly sensitive, field-deployable diagnostics coupled with robust surveillance systems", a principle that guides our innovation and strategic direction.
This content is for informational purposes only and does not constitute medical advice.
For detailed product specifications and instructions for use, please refer to the official product documentation provided with the kit.
This is the last article
-
Malaria Pf Ag Rapid Test Kit - Quick & Accurate Detection
NewsAug.11,2025
-
Accurate Cardiac Marker CK-MB Rapid Test for Quick Results
NewsAug.10,2025
-
Premium Empty ABS Plastic Cassette for Test Strips
NewsAug.09,2025
-
Sterile Urine Cup: Accurate Specimen Collection for Labs & Home
NewsAug.08,2025
-
Malaria Pf/Pan Ag Rapid Test Kit for Fast, Accurate Diagnosis
NewsAug.07,2025
-
Rapid Canine Corona Test: Fast & Accurate Results
NewsAug.06,2025

