PRISES Biotechnology は、体外診断試薬 (IVD) および医療機器の開発、製造、取引に従事する研究開発ベースのメーカーであり、NMPA (CFDA) から IVD 製品の製造および取引を承認され、ISO 13485 の品質システムの下で運営されています。の製品が CE マークを取得しています。
だいたい
PRISES
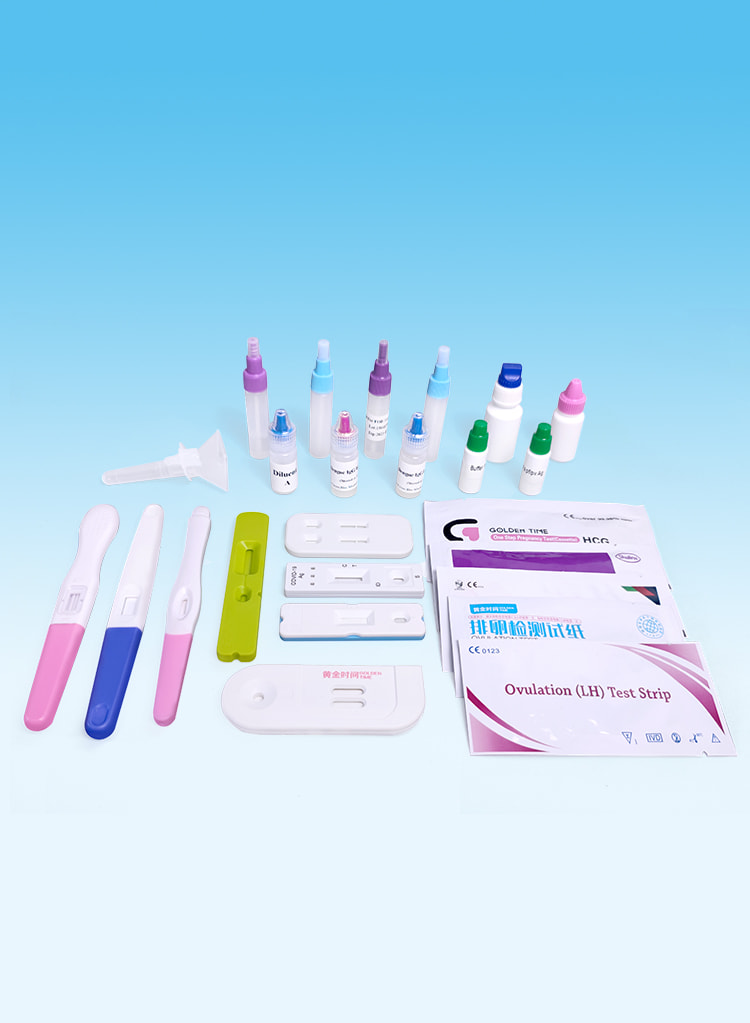
Our factory is founded in 2012 and located in Gaobeidian City, which is near Xiongan New Area and Beijing. It covers an area of 3,000 square meters, including class 1000,000 clean workshop with 1000 square meters, class 10 thousands of microbiological testing room with 500 square meters, well-equipped quality inspection rooms, research and development laboratories, etc.
-

10+
Business growth
10+
Business growthEstablished 11 years ago, the company has become a well-established and dynamic business through continuous efforts and improvements.
詳細を見る -

100000
Clean workshop
100000
Clean workshopClass 100,000 clean workshop 1000 square metres, Class 10,000 microbiological test room 500 square metres, fully equipped quality control room, R&D laboratory, laboratory, etc.
詳細を見る -

30+
Research staff
30+
Research staffThe company has a research and development team of 35 people who drive the technology and support the development of new products for the company.
詳細を見る -

20+
Consultant
20+
ConsultantWe provide the highest level of service to our customers, whether it's answering questions, solving problems or providing valuable advice, we deal with it promptly.
詳細を見る -

100+
Professionals
100+
ProfessionalsThe company has a large team of professionals responsible for managing, coordinating and controlling the operations of the various functions.
詳細を見る
ニュースと情報
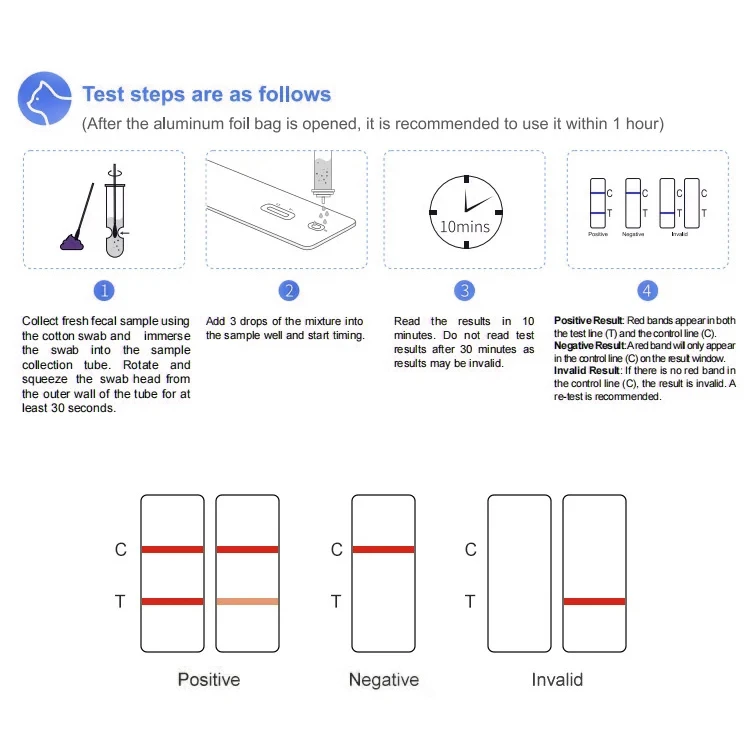
The Benefits of Rapid Vet Test Kits
The advent of vet diagnostic test kit technology has transformed how pet owners monitor their animals' health.
LH Strip Test Accuracy
When it comes to tracking ovulation, lh ovulation test, lh strip test, and urine ovulation test are among the most popular tools used by women trying to conceive.
Importance of Ovulation Prediction Test Kits
For women trying to conceive, understanding their fertile window is crucial.