7월 . 26, 2025 01:01 Back to list
High-Quality Nasal Swab for Accurate Testing – Fast Results
Official Website



1. Nasal Swab Market Overview & Industry Trends
In recent years, demand for nasal swab devices has surged globally, driven by the proliferation of rapid diagnostic needs (especially in response to pandemics), increased prevalence of respiratory diseases, and the expansion of molecular testing platforms. According to industry forums and scholarly reports, the nasal test swab market is expected to achieve a compound annual growth rate (CAGR) of over 6% from 2024-2030.
- Widespread adoption in clinical, forensic, and research settings.
- Advancements in swab materials (notably, nylon flocked swabs) for improved sample release and user comfort.
- Increased emphasis on ergonomics, sample integrity, and compatibility with modern molecular platforms.
- Stringent regulatory standards across EU, US, and APAC regions enhance reliability.
Gaobeidian PRISES Biotechnology Co., Ltd remains at the forefront, consistently integrating novel ergonomic design, quantitative capabilities, and multi-platform usability to ensure a superior diagnostic experience.
2. Nasal Swab Core Parameters: At a Glance
| Parameter | Common Range/Spec | Description |
|---|---|---|
| Material | Nylon flocked, Polyester, Foam, Rayon | Provides optimal sample release and patient comfort. |
| Sterility Assurance | ≥10-6 SAL | Gamma/E-beam sterilized for clinical safety. |
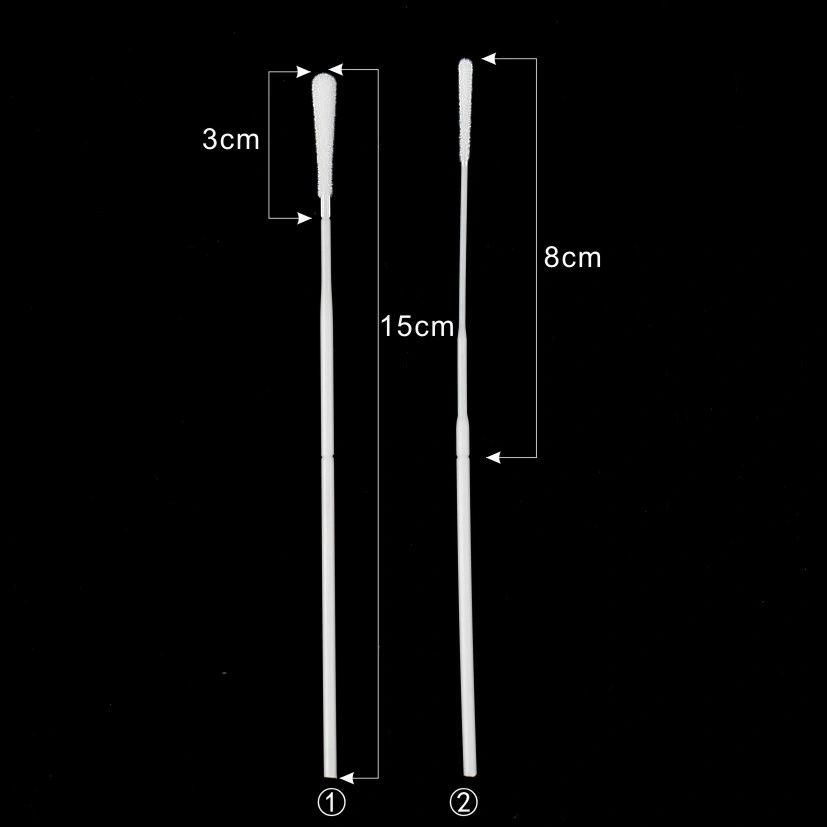
| Swab Head Size | 2.5-4.5 mm | Optimized for nasal passage and cell collection efficiency. |
| Breakpoint Position | 30–80 mm from tip | Facilitates easy sample transfer into transport tube. |
| Compatibility | PCR, RT-PCR, EIA, Antigen Tests, Bacterial/Viral Culture |
Multi-platform diagnostic applications. |
| Sampling Efficiency | 70–95% | Higher with flocked swabs (vs. cotton/polyester). |
| Elution Release Time | <10 sec | For rapid transfer of sample into media. |
3. PRISES Sterile Nasal Swab Nylon: Technical Specifications & Innovations
Sterile Nasal Swab Nylon by Gaobeidian PRISES Biotechnology Co., Ltd stands as a benchmark in clinical sample collection.
- Ergonomic & anatomical design: User-friendly for enhanced comfort and precise sample retrieval.
- Soft nylon flocking: Ensures efficient cell detachment and rapid absorption.
- Safe breakpoints: Multiple breakpoint options adapt to diverse sampling/situational needs.
- Automatic elution: Swift and reproducible sample release into transport media, minimizing viral/cellular loss.
- Quantitative capabilities: Consistent uptake and transfer for accurate, sensitive diagnostics.
- Multi-platform compatibility: PCR, antigen, DFA, EIA, and cell morphology diagnostics supported.
4. Application Scenarios
Gaobeidian PRISES Biotechnology Co., Ltd offers nasal swab products tailored for wide-ranging diagnostic and research deployments:
- Clinical diagnostics: Influenza, COVID-19, RSV, and other respiratory virus detection.
- Rapid antigen testing: Point-of-care screening in clinics, field hospitals, and airports.
- Forensic applications: Human identification and trace evidence acquisition.
- Microbiological studies: Sampling for bacteriological and virological cultures.
- Cytology & genetic research: Collection of epithelial and mucosal cells for DNA/RNA analysis.
5. Professional Q&A: Nasal Swab Technical Terminologies
A1: High-purity flocked nylon fibers, providing optimized sample collection and rapid elution.
Q2: What are the available swab head specifications?
A2: Swab head diameters range typically from 2.5–4.5mm, with lengths of 15–25mm, ideal for various age groups and sampling sites.
Q3: What are breakpoints in a nasal swab?
A3: Engineered points (usually 30-80mm from tip) allowing clean, safe detachment after sampling for tube transport.
Q4: Are PRISES nasal swabs compatible with all transport media?
A4: Yes, PRISES swabs are validated with common viral, bacterial, and molecular transport media (UTM, VTM, saline, lysis buffer).
Q5: How is sterility achieved and ensured?
A5: Sterilization via gamma irradiation or electron beam to maintain a sterility assurance level (SAL) of at least 10-6.
Q6: What standards or certifications do PRISES nasal swab for sale meet?
A6: CE marking (IVD), FDA registration, ISO 13485:2016, and Chinese National Medical Products Administration (NMPA) compliance.
Q7: Are PRISES nasal test swab products suitable for both adults and children?
A7: Absolutely; swab shapes, head sizes, and shaft flexibility options accommodate a broad patient demographic for safe, efficient sampling.
6. Why Choose PRISES for Nasal Swab Solutions?
- Proven nasal swab performance, verified by leading laboratories and academic research (Oxford Academic).
- Continuous investment in R&D for ergonomics, safety, and automation technologies.
- Extensive quality control, from sterile manufacturing to post-market surveillance.
- Global distribution and technical support network.
- Responsibly produced, in full compliance with international medical device standards.
Tel: 0086-(0)312-2930588
Mobile: 0086-15910623759
Email: export@prises.cn
Address: No.136, Shiji West Road, Gaobeidian City, 074000, Hebei Province, P.R. China
Website: https://www.prisesbio.com
7. Global Demand & Nasal Test Swab: Search, Data, and Market Insights
| Year | Global Demand (Units in millions) | Search Volume | Main Usage |
|---|---|---|---|
| 2015 | 180 | 12,000 | Influenza/PCR diagnostics |
| 2018 | 320 | 18,500 | Microbiology, Forensics |
| 2020 | 1,500 | 185,000 | Pandemic Screening – COVID-19 |
| 2023 | 940 | 82,000 | Rapid antigen, Genotyping |
| 2024 est* | 850 | 77,000 | Antigen/PCR/DNA, Forensic |
8. References & Further Reading
- ScienceDirect, "Advancements in Sampling Swab Technology"
- NCBI Forum, "Diagnostic Sampling and Swab Selection"
- Frontiers in Plant Science, "Flocked vs. Traditional Swabs"
- Oxford Academic, "Saliva, Nasal, and Oropharyngeal Swabs in Viral Diagnostics"
- Mordor Intelligence, "Viral Transport Media Market Trends"
This is the last article
-
High-Quality Nasal Swab for Accurate Testing – Fast Results
NewsJul.26,2025
-
One Step LH Ovulation Test Kit - Accurate & Easy At-Home Fertility Tracking
NewsJul.25,2025
-
Sterile Urine Cup for Accurate Specimen Collection | Leak-Proof Design
NewsJul.24,2025
-
High Quality Cassette Lateral Flow for Accurate Testing Solutions
NewsJul.23,2025
-
Malaria PF / PAN AG Rapid Test – Accurate & Fast Malaria Diagnosis
NewsJul.22,2025
-
Accurate LH Ovulation Test Strips for Easy Fertility Tracking
NewsJul.21,2025

