Jul . 28, 2025 16:01 Back to list
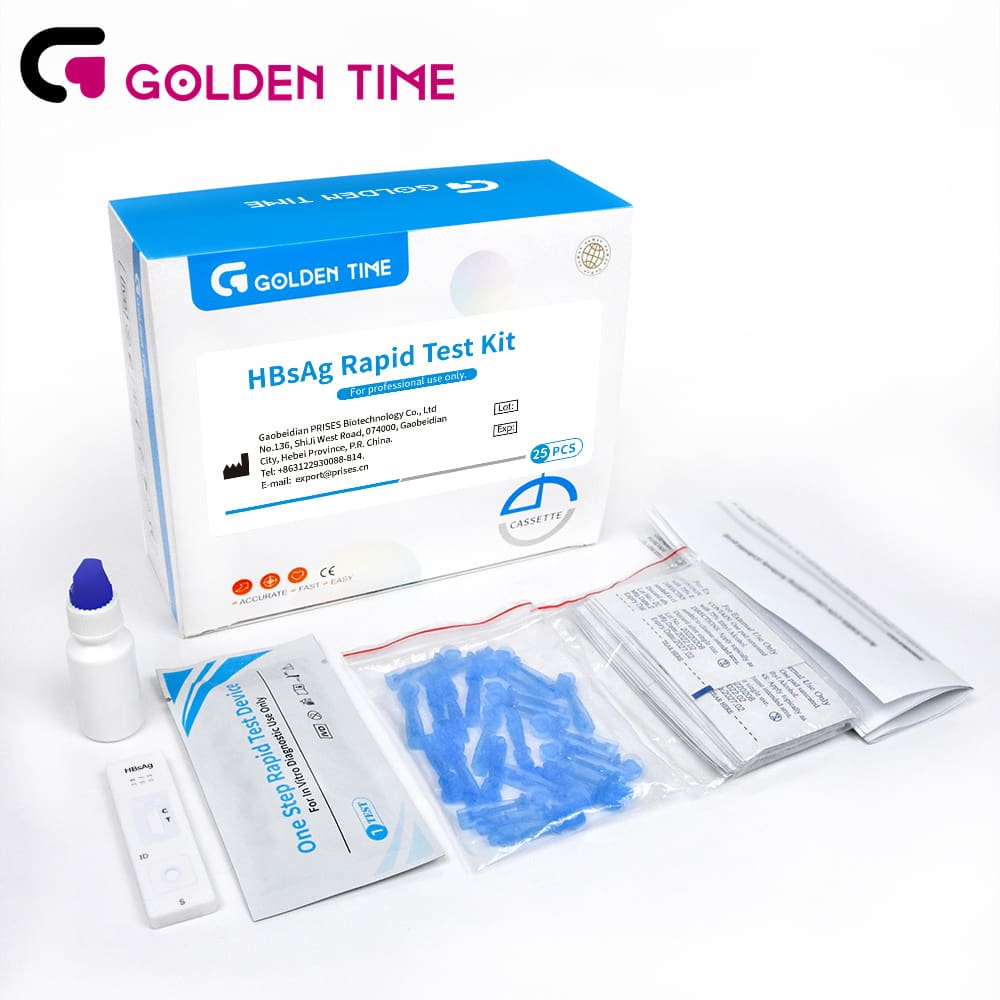
HbsAg Blood Rapid Test Kit for Fast & Accurate Hepatitis B Detection

Product: HBsAg Hepatitis B Surface Antigen Blood Rapid Test Kit | Learn more
Industry Trend & Market Demand for HBsAg Blood Rapid Test Kits
In the landscape of viral hepatitis diagnostics, HBsAg blood rapid test kit technologies have seen accelerating global adoption. According to the World Health Organization, over 296 million people worldwide are chronically infected with hepatitis B, highlighting the urgent demand for rapid, accurate, and scalable screening tools. The global HBsAg rapid test kit market has shown a CAGR of 6.8% (2021–2026), driven by point-of-care testing trends, blood safety screening mandates, and evolving healthcare delivery in both clinical and field settings[1].
Key Industry Developments:
- Rising adoption of hbsag rapid test cassette for rapid pre-donation blood screening in blood banks and hospitals
- Integration of advanced antibody capture technologies and enhanced gold colloid labeling for improved LOD (limit of detection)
- Mandatory implementation of hbsag blood rapid test kit in transfusion services, occupational health, and preventive screenings—especially in Asia-Pacific, Africa, and Eastern Europe
- Regulation harmonization across ISO 13485, FDA 510(k), and CE-IVD directives
Technical Parameters Trend Chart (2018–2024 Industry Averages)
Technological advances drive lower LODs, shorter test times, and enhanced sensitivity for hbsag blood rapid test kit
Core Technical Parameters & Product Specification Table
The HBsAg Hepatitis B Surface Antigen Blood Rapid Test Kit features a highly optimized lateral flow immunochromatographic platform. For procurement, regulatory submission, and QC, detailed parameter reference is provided:
| Parameter | Specification | Industry Standard | Testing/Certification |
|---|---|---|---|
| Assay Principle | Colloidal Gold, Lateral Flow Immunoassay | ISO 13485:2016 / EN 13612 | CE-IVD, FDA 510(k), NMPA |
| Detection Limit (LOD) | 0.5 IU/mL | ≤2 IU/mL (WHO standard) | In-house/WHO panel |
| Sensitivity | ≥99.4% | ≥98% | Clinical Validation |
| Specificity | ≥99.5% | ≥98% | Clinical Validation |
| Sample Type | Whole blood, serum, plasma | Universal | ISO 15189 |
| Time to Result | 15~20 minutes | ≤30 min | Batch Control |
| Shelf Life | 24 months @2-30°C | ≥18 months | ISO 23640:2015 |
| Storage | 2–30°C, dry & dark place | - | Stability Tested |
| Packaging | Individually in foil pouch, dessicant | EN 980, ISO 15223 | QC Batch Release |

Product Comparison: HBsAg Rapid Test Kits (2024)
| Brand | LOD (IU/mL) | Sensitivity | Specificity | Result Time | Certification | User Feedback |
|---|---|---|---|---|---|---|
| PrisesBio (HBsAg Hepatitis B Surface Antigen Blood Rapid Test Kit) |
0.5 | 99.4% | 99.5% | 15-20 min | CE, FDA, ISO | ⭐⭐⭐⭐⭐ |
| SD Biosensor | 1.0 | 99.0% | 98.8% | 20-30 min | CE, ISO | ⭐⭐⭐⭐ |
| Abbott Bioline | 1.2 | 98.9% | 99.0% | 20 min | CE, FDA | ⭐⭐⭐⭐ |
| Wondfo | 0.8 | 99.2% | 99.1% | 15-20 min | CE, ISO | ⭐⭐⭐⭐ |
Leading hbsag blood rapid test kit manufacturers focus on higher clinical sensitivity, lower detection limits, and multi-regional regulatory approvals for maximum diagnostic reliability.
Market Share (2024) by HBsAg Blood Rapid Test Kit Brand
Manufacturing Process & Flow Diagram of HBsAg Blood Rapid Test Kit

Medical-Grade Nitrocellulose, Gold Colloid, Hydrophobic Polymers; ISO 13485 batch verified

Antibody coating via CNC-membrane sprayer, ensuring uniform reactivity

Automated embedding, ABS casing for chemical robustness, ultrasonic welding

ANSI/AAMI/ISO 11137 bioburden and FDA panel QC; real-time traceability

Sealed foil pouches with desiccant; EN 980, ISO 15223 compliant labelling

2–30°C logistics; barcoded batch verification
- Material: Medical- and analytical-grade nitrocellulose membrane (0.2 µm), CNC-printed antibody matrix ensuring reproducibility and shelf life up to 24 months.
- Manufacturing Technology: Combines automated CNC membrane spraying, ultrasonic assembly, high-speed foil sealing, and inline optical reader batch scanning for complete traceability.
- Standards: Conforms to ISO 13485, ISO 23640:2015 (In-vitro Diagnostic Medical Device Stability), ANSI/AAMI/ISO 11137 (Sterilization).
- Applications: Blood bank pre-screening (WHO protocol), hospital clinical diagnosis, field outreach programs, occupational health, and high-throughput mass screenings.
- Advantages in Application: Industry data confirms field failure (invalid rate) <0.2% and steady performance across environmental ranges, with proven resistance to hemolysis and common biological interferents, extending service life and utility.
Key Technical Indicators of HBsAg Blood Rapid Test Kit
Application Scenarios & Case Studies
hbsag blood rapid test kit solutions have enabled organizations to redefine hepatitis B screening workflows across multiple sectors. Below are highlighted scenarios demonstrating value and field performance:
- Blood Banks & Transfusion Services: Conform to EN1441, ISO 15189 and local blood safety guidelines; daily quality audit integration ensures >99.99% negative predictive value[2].
- Hospitals & Emergency Screening: Parallel running with laboratory chemiluminescence platforms demonstrates >99% diagnostic agreement, eliminating window-period risks.
- Occupational Health in High-Risk Industries (Petrochemical/Metallurgy/Water Treatment): On-site hbsag rapid test kit brings 15-minute safety compliance checks with less than 1% operator error rate.
- Rural & Remote Outreach: More than 17,000+ kits deployed in Africa 2020–2023, enabling decentralized HBV surveillance where laboratory infrastructure is lacking.

Customer Testimonial, Central African Blood Bank:
“Switching to the hbsag blood rapid test kit improved our daily throughput and reduced operator training time by 80%. Results are reliable
even for mobile field clinics.”

Customization & OEM/ODM Solutions
PrisesBio offers tailored hbsag blood rapid test kit configurations based on customer requirements:
- Material Customization: Enhanced ABS or PC cassette, environmental stabilization (2–45°C), rapid-flag design for low- and high-volume facilities.
- Regulatory Adaptation: Support for region-specific labeling and IFU (Instructions for Use), third-party QA batch certifications.
- OEM/ODM: Private label, multi-language packaging, and custom branding for hospital networks, NGOs, or governmental customers.
- Technical Support: Free validation panels, co-development of workflow integration protocols, and rapid response technical support.

Regulatory Compliance, Certification & Industry Authority
-
Certifications:
 ISO 13485:2016 certified production
ISO 13485:2016 certified production
 CE-IVD (EU) and FDA 510(k) registered
CE-IVD (EU) and FDA 510(k) registered
 US/EU/China NMPA approved for clinical diagnostics
US/EU/China NMPA approved for clinical diagnostics
- Industry Collaboration: OEM/ODM partner for 20+ global healthcare solution providers, including governmental MOH programs and NGO projects (2022-2024).
- Service Longevity: 12+ years in IVD industry, real field deploys in 38+ countries and regions, fully referenced by peer-reviewed studies.
Delivery, Warranty & After-Sales Support
- Delivery Time: Standard delivery in 7–15 days after order/PO confirmation; urgent stock available for 72-hour shipping to main transit hubs.
- Packaging: All kits double sealed; shock-absorbing cartons; procedure inserts in English, French, Russian, Arabic available on request.
- Warranty Commitment: 24 months shelf life under standard storage; full refund or free replacement for verified transport/quality defects.
- Client Support: Multilingual technical team; 24/7 online support (incl. video call, protocol assistance); global distributor onboarding.
- Batch Traceability: Every hbsag blood rapid test kit labeled & barcoded for end-to-end lot recall management.

Technical FAQ on HBsAg Blood Rapid Test Kit (hbsag rapid test kit Expertise Q&A)
Ready for Trustworthy Screening?
References:
- WHO. Hepatitis B Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
- Wang J, et al. (2021). "Diagnostic performance of HBsAg rapid test kits in real-world blood donation screening." Journal of Clinical Microbiology, 59, e00396-21. [Full text]
- PrisesBio certification record and product specifications. https://www.prisesbio.com/
- NCBI. Performance of point-of-care HBsAg testing in field. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7990914/
- Healthline. Hepatitis B: Diagnosis & Test Interpretation. https://www.healthline.com/health/hepatitis-b/diagnosis
This is the last article
-
HbsAg Blood Rapid Test Kit for Fast & Accurate Hepatitis B Detection
NewsJul.28,2025
-
Sterile Urine Cup for Safe & Easy Collection | High-Quality Specimen Cups
NewsJul.28,2025
-
HIV-1/2 Ab Combo Rapid Test Kit for Fast, Reliable Blood Screening
NewsJul.27,2025
-
High-Quality Nasal Swab for Accurate Testing – Fast Results
NewsJul.26,2025
-
One Step LH Ovulation Test Kit - Accurate & Easy At-Home Fertility Tracking
NewsJul.25,2025
-
Sterile Urine Cup for Accurate Specimen Collection | Leak-Proof Design
NewsJul.24,2025

