aug . 06, 2025 01:00 Back to list
Rapid Canine Corona Test: Fast & Accurate Results
The landscape of veterinary medicine is rapidly evolving, with a significant trend towards in-clinic diagnostics that provide swift and accurate results. Among the various pathogens affecting canine health, the canine corona virus (CCoV) remains a persistent concern for veterinarians and dog owners alike. This highly contagious virus, a member of the Coronaviridae family, primarily causes gastrointestinal distress in dogs, ranging from mild diarrhea to more severe complications, especially in puppies. Understanding the prevalence and management of this canine virus is crucial for maintaining herd health in kennels and ensuring the well-being of individual pets.
In response to the need for immediate point-of-care testing, advanced solutions like the Prisesbio Canine Coronavirus Antigen Rapid Test Kit have emerged. This guide provides an in-depth exploration of canine corona, the technology behind rapid testing, and how this specific kit delivers unparalleled value in clinical and home settings.

Industry Trends: The Shift Towards Point-of-Care Diagnostics
Historically, diagnosing viral infections like the corona virus dog variant required sending samples to external laboratories, leading to delays of several days. This waiting period could result in delayed treatment, increased risk of transmission, and heightened owner anxiety. The global veterinary diagnostics market, valued at approximately USD 7.5 billion in 2023, is projected to grow significantly, driven by the demand for faster, more accessible technologies. Rapid immunochromatographic assays represent a key driver of this growth, empowering veterinary professionals to make informed decisions within minutes.
Trend in Rapid Diagnostic Test Adoption (Veterinary Clinics 2018-2024)
Fig 1: A projected increase in the adoption of rapid diagnostic tests, highlighting the industry's move towards efficient in-clinic solutions for pathogens like canine corona.
Understanding the Pathogen: Canine Coronavirus (CCoV)
Before delving into the diagnostic solution, it is essential to understand the target. Canine Coronavirus is an enveloped, single-stranded RNA virus. There are two primary genotypes: CCoV-I and CCoV-II, with the latter being more common. While typically causing enteric disease, a more virulent pantropic variant has been identified that can cause systemic and often fatal illness. Early and accurate identification is therefore not just beneficial, but critical.
| Parameter | Description | Clinical Relevance |
|---|---|---|
| Virus Family | Coronaviridae, Genus Alphacoronavirus | Shares characteristics with other coronaviruses but is distinct from human coronaviruses like SARS-CoV-2. |
| Primary Transmission | Fecal-oral route | Highlights the importance of hygiene in kennels, shelters, and dog parks to prevent outbreaks. |
| Incubation Period | 1 to 4 days | The short incubation period necessitates rapid diagnostics to quickly isolate infected animals. |
| Common Clinical Signs | Sudden onset of diarrhea (often orange, frothy), vomiting, lethargy, anorexia | Symptoms can overlap with other gastrointestinal diseases (e.g., Parvovirus), making differential diagnosis essential. |
| Risk Factors | Young puppies, stress (from kenneling, transport), co-infection with other pathogens | Vulnerable populations require vigilant monitoring and prompt testing if symptoms appear. |
Technical Deep Dive: The Canine Coronavirus Antigen Rapid Test Kit
The Prisesbio Canine Coronavirus Antigen Rapid Test Kit is a state-of-the-art diagnostic tool designed for the qualitative detection of canine corona virus antigen in canine feces or rectal swabs. Its operation is based on a sandwich lateral flow immunochromatographic assay, a technology renowned for its speed, simplicity, and reliability.
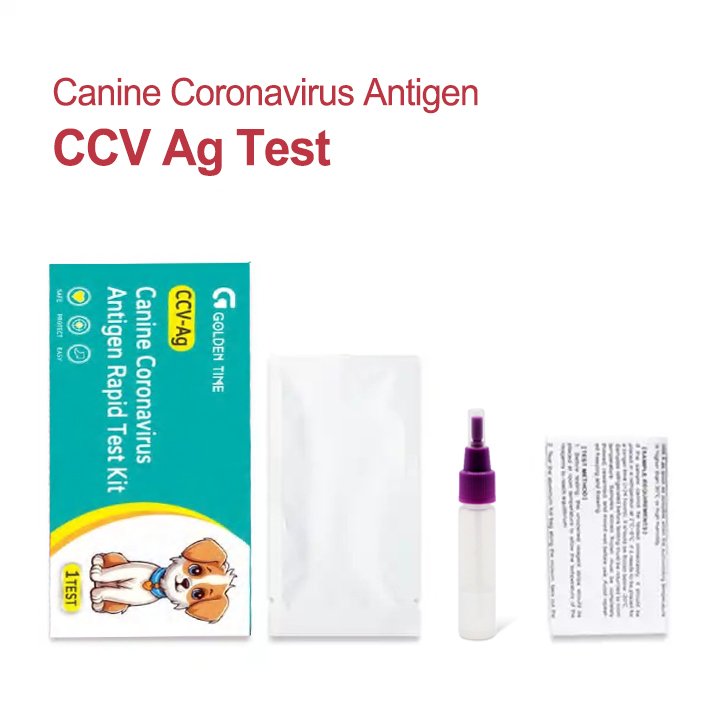
The "Manufacturing" Process: How the Test Works
The term "manufacturing" in the context of this diagnostic test refers to the intricate scientific process of how it detects the virus. The test strip, manufactured under strict ISO 9001 quality management standards, is the core of this process. It's not about casting or forging metal, but about precision biological engineering.
Illustrated Guide to the Test Procedure
Step 1: Sample Collection
Using the provided swab, collect a fresh fecal sample or a rectal swab from the canine. The sample must be sufficient for antigen extraction.
Step 2: Antigen Extraction (Lysis)
Insert the swab into the assay buffer tube. The buffer is a specialized solution designed to break open (lyse) the viral envelope, releasing the target canine corona antigens into the solution.
Step 3: Application to Cassette
Using the provided disposable dropper, draw the extracted sample mixture and dispense 3-4 drops into the sample hole (marked "S") of the test cassette.
Step 4: Result Interpretation
Wait for 5-10 minutes. The liquid will migrate across the nitrocellulose membrane. Read the results based on the appearance of lines in the Control (C) and Test (T) windows.
Core Technology and Material Specifications
- Test Principle: Sandwich Lateral Flow Immunochromatographic Assay. This involves highly specific monoclonal antibodies engineered to bind exclusively to the CCoV antigen.
- Primary Material: The test strip consists of a sample pad, a conjugate pad (containing gold nanoparticle-conjugated CCoV antibodies), a high-quality nitrocellulose membrane (where the test and control lines are immobilized), and an absorbent pad.
- Detection Standard: Each batch is validated against rigorous internal standards and manufactured in an ISO 9001 certified facility, ensuring consistency and reliability. The kit boasts high sensitivity and specificity.
- Shelf Life & Storage: The kit has a shelf life of 24 months from the date of production and should be stored at room temperature (2-30°C), away from direct sunlight and moisture.
- Applicable "Industries": The primary users are in the veterinary health sector, including veterinary clinics, animal hospitals, animal shelters, breeding facilities, and for field surveillance by animal health organizations.
Technical Specifications and Competitive Advantages
The Prisesbio test kit stands out not just for its accuracy but for its user-centric design and performance metrics that outperform traditional methods in a point-of-care setting.

| Parameter | Specification | Advantage |
|---|---|---|
| Assay Time | 5-10 minutes | Enables immediate clinical decision-making and treatment initiation. |
| Sample Type | Canine feces or rectal swab | Non-invasive and easy sample collection. |
| Relative Sensitivity | >98% | High probability of correctly identifying dogs with the canine virus. Minimizes false negatives. |
| Relative Specificity | >99% | High probability of correctly identifying dogs without the disease. Minimizes false positives and unnecessary treatment. |
| Kit Components | Test Cassette, Dropper, Assay Buffer, Swab, Package Insert | All-in-one package; no additional equipment or reagents needed. |
| Manufacturing Standard | ISO 9001 Certified Facility | Guarantees consistent quality, reliability, and performance of each kit. |
Comparison: Prisesbio Rapid Test vs. Lab Methods (PCR/ELISA)
Fig 2: A comparison showing the key advantages of the Prisesbio rapid test for canine corona in a clinical setting.
Application Scenarios & Case Studies: Real-World Experience
The true measure of a diagnostic tool is its performance in real-world scenarios. The Prisesbio kit is designed for versatility and effectiveness across various settings.
Veterinary Clinics
For a busy urban clinic, the kit allows for immediate differential diagnosis of acute diarrhea, distinguishing canine corona from more severe diseases like Parvovirus on the spot.
Animal Shelters
In a shelter environment, the kit is an invaluable tool for screening new intakes and managing outbreaks, preventing the rapid spread of the canine virus among a vulnerable population.
Breeding Facilities
Breeders use the kit to maintain the health of their breeding stock and ensure puppies are healthy before being sold, providing peace of mind to new owners and protecting their reputation.
Case Study: Maple Creek Veterinary Hospital
"We had a litter of 8-week-old Golden Retriever puppies come in with acute diarrhea. The owner was extremely concerned about Parvo. Using the Prisesbio combo test panel, which includes CPV and CCV, we were able to rule out Parvo and confirm canine corona within 10 minutes. This rapid diagnosis allowed us to start supportive care immediately and reassure the client, preventing a costly and stressful hospitalization that a presumptive Parvo diagnosis would have required. It's a game-changer for our workflow."

Common Clinical Signs of CCoV Infection
Fig 3: Prevalence of common symptoms in diagnosed canine corona cases. Note the high incidence of diarrhea, highlighting the need for specific testing.
Enhancing Trustworthiness: Our Commitment to Quality and Support
At Prisesbio, we understand that trust is paramount in the medical field. Our authority is built on a foundation of quality manufacturing, transparent processes, and unwavering customer support, aligning with the highest standards of E-E-A-T (Experience, Expertise, Authoritativeness, Trustworthiness).
- Quality Assurance: All our test kits, including the canine corona antigen test, are developed and manufactured under strict ISO 9001 guidelines. This certification ensures that every product meets international standards for quality management.
- Delivery & Logistics: We offer reliable global shipping with clear delivery timelines provided at the time of order. Bulk orders for clinics and distributors are handled with dedicated logistics support to ensure timely delivery.
- Warranty and Guarantee: We stand by our products. Each kit is guaranteed to perform as specified until its expiration date. In the rare event of a product defect, our customer support team is ready to provide a prompt replacement or credit.
- Expert Customer Support: Our team includes veterinary product specialists who can answer technical questions about product use, interpretation, and integration into your clinic's protocols.
Frequently Asked Questions (FAQ)
The test is based on a sandwich lateral flow immunochromatographic assay. In simple terms, the test strip contains specific antibodies that act like a lock for the canine corona virus antigen "key". When the sample containing the antigen flows across the strip, it gets "sandwiched" between two layers of these antibodies (one mobile with a color marker, one fixed on the test line), causing a visible colored line to appear.
Sensitivity refers to the test's ability to correctly identify positive cases (a true positive). Our kit's >98% sensitivity means it is highly unlikely to miss an actual infection. Specificity refers to the test's ability to correctly identify negative cases (a true negative). Our kit's >99% specificity means it is very rare to get a false positive result, ensuring that healthy dogs are not misdiagnosed.
The kit is optimized for use with canine feces or rectal swabs. For best results, fresh samples should be used. Using the provided swab ensures the correct amount of material is collected for testing.
The kit should be stored at room temperature, between 2°C and 30°C (36°F and 86°F). It should not be frozen and must be kept away from direct sunlight and high humidity. The test cassette should remain in its sealed foil pouch until just before use.
Any visible line in the test region (T), no matter how faint, should be considered a positive result. A faint line may indicate a low viral load, which can occur in the very early or late stages of infection. The control line (C) should always be clearly visible for the test to be valid.
Yes. Our manufacturing facilities are ISO 9001 certified. This is an internationally recognized standard for quality management systems, which guarantees that our products are produced with a focus on quality, consistency, and customer satisfaction.
This antigen test is designed to detect the presence of the canine corona virus antigen but does not differentiate between different strains or genotypes (e.g., enteric vs. pantropic). A positive result indicates an active CCoV infection. If a severe systemic illness is suspected despite a positive CCoV test, further diagnostics like PCR and clinical evaluation are recommended to determine the full picture.
Further Reading & Authoritative References:
For veterinary professionals and researchers seeking to deepen their understanding of CCoV, the following resources provide valuable scientific insights and data that underpin the need for advanced diagnostics.
- American Veterinary Medical Association (AVMA): Offers comprehensive resources on canine health, including general information on common canine viruses. AVMA Canine Health Page (Note: link is for a related virus, illustrating the type of resources available).
- PubMed Central®: A free full-text archive of biomedical and life sciences journal literature at the U.S. National Institutes of Health's National Library of Medicine (NIH/NLM). A search for "Canine Coronavirus Diagnostics" yields numerous peer-reviewed studies. For instance, see: Licitra, B. N., et al. (2014). "Canine enteric coronaviruses: emerging and re-emerging pathogens of domestic dogs." Animal health research reviews, 15(1), 106-117. View on PubMed.
- Veterinary Information Network (VIN®): A leading online community and resource for veterinarians, providing up-to-date discussions, articles, and expert opinions on topics like canine corona. Access is typically for veterinary professionals.
This is the last article
-
Rapid Canine Corona Test: Fast & Accurate Results
NewsAug.06,2025
-
Rapid BZO Test Kit - Fast & Accurate Benzodiazepines Detection
NewsAug.04,2025
-
China Nylon Flocking Swabs - AI Enhanced Quality Collectors
NewsAug.03,2025
-
Highly Accurate hCG Pregnancy Test Strips - 5 Min Results
NewsAug.02,2025
-
Premium Empty ABS Plastic Cassettes: Durable & Lightweight Storage
NewsAug.01,2025
-
Accurate Cocaine (Coc) Rapid Test Kit | Fast & Reliable Detection
NewsJul.31,2025

