7 月 . 25, 2025 01:01 Back to list
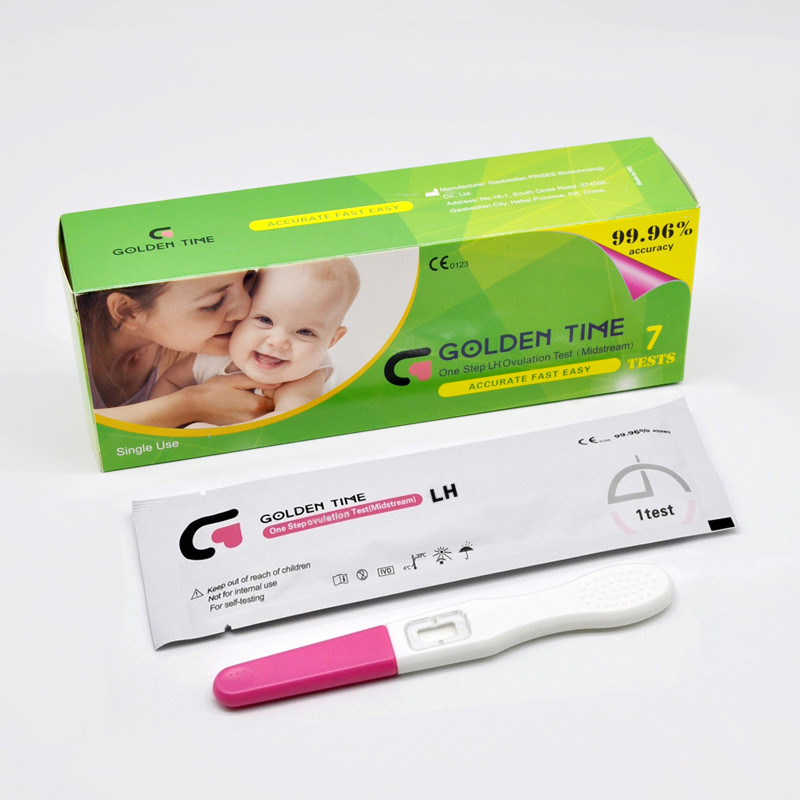
One Step LH Ovulation Test Kit - Accurate & Easy At-Home Fertility Tracking
In today's healthcare diagnostics industry, accurate ovulation prediction is essential for reproductive health and fertility planning. The one step LH ovulation test, represented by Urine LH Ovulation Test Midstream, is at the forefront of biotechnology, offering reliable and user-friendly solutions for both clinical and self-detection scenarios. Produced by Gaobeidian PRISES Biotechnology Co., Ltd, this advanced product is transforming how women monitor ovulation cycles worldwide.



Industry Trends: Growing Demand for Rapid and Reliable Ovulation Tests
The global one step LH ovulation test market is projected to grow at a CAGR of 7.2% from 2023–2028[1]. This surge is propelled by the increasing awareness of women’s reproductive health, the convenience of at-home self-testing, and the demand for fast, accurate, and non-invasive methods. According to ScienceDirect, immunochromatographic technology remains the gold standard for ovulation prediction due to its high sensitivity, specificity, and ease of use.
| Parameter | Description | Industry Standard | Gaobeidian PRISES Value |
|---|---|---|---|
| Detection Principle | Immunochromatographic One Step Assay | Lateral Flow | Lateral Flow |
| Target Analyte | Human Luteinizing Hormone (hLH) | hLH | hLH |
| Sample Type | Urine | Urine | Urine |
| Result Time | Within 5 Minutes | 5–10 Minutes | ≤5 Minutes |
| Cutoff Sensitivity | 25 mIU/mL | 20–40 mIU/mL | 25 mIU/mL |
| Specificity | ≧ 99% | ≧ 98% | ≧ 99% |
| Format | Midstream | Strip, Midstream, Cassette | Midstream |
| Storage | 2–30°C | 2–30°C | 2–30°C |
| Shelf Life | 24 months | 18-24 months | 24 months |
Cutting-edge Technology: Gaobeidian PRISES Urine LH Ovulation Test Midstream
Urine LH Ovulation Test Midstream from Gaobeidian PRISES Biotechnology Co., Ltd is an immunochromatographic, one-step in vitro diagnostic device engineered to qualitatively detect hLH surges in urine, which signal imminent ovulation. As described in industry forums such as LabRoots, the core lateral flow technology in these test kits offers outstanding accuracy and reliability while ensuring easy self-administration.
Product Highlights:
- High sensitivity, precise to 25 mIU/mL hLH
- Quick results within 5 minutes for maximal user convenience
- Clear and easy-to-interpret result lines, minimizing user error
- Innovative sample pad technology for effective urine absorption
- User-friendly design enables hygienic, direct midstream use
Application Scenarios of One Step LH Ovulation Test
The one step LH ovulation test and urine LH ovulation test midstream have broad applications spanning from personal fertility management to clinical reproductive medicine. Specific use-cases include:
- At-home Fertility Monitoring: Enables women trying to conceive to pinpoint their fertility window for optimal pregnancy outcomes.
- Gynecology Clinics: Offers fast diagnostics to support ovulation cycle assessment and fertility treatments.
- IVF and ART Support: Essential in assisted reproductive technologies (ART) to schedule oocyte retrieval and embryo transfer at the optimal time.
- Hormonal Disorder Screening: Helps in evaluating disorders such as PCOS, anovulation, and infertility.
Contact Gaobeidian PRISES Biotechnology Co., Ltd
Company: Gaobeidian PRISES Biotechnology Co., Ltd
Website: https://www.prisesbio.com
Tel: 0086-(0)312-2930588
Email: export@prises.cn
Mobile: 0086-15910623759
Address: No.136, Shiji West Road, Gaobeidian City, 074000, Hebei Province, P.R. China
Data Visualization: Technical Parameters & Market Insights
Technical Sensitivity Trends of One Step LH Ovulation Test
LH Test Accuracy: Leading Brands Comparison
Market Share: Urine LH Ovulation Test Midstream Formats
Shelf Life Comparison of Rapid Ovulation Tests
Technical Specifications of the Urine LH Ovulation Test Midstream
| Parameter | Specification | PRISES Test Value |
|---|---|---|
| Detection Principle | Immunochromatography (Lateral Flow) | Yes |
| Analyte | Luteinizing Hormone (hLH) | 25 mIU/mL detection |
| Format | Midstream | Midstream |
| Specimen Type | Urine | Urine |
| Result Time | ≤ 5 minutes | 4–5 minutes |
| Accuracy | ≧98% | ≧99% |
| Storage | 2–30°C | 2–30°C |
| Shelf Life | 24 months | 24 months |
| Certifications | FDA, CE, ISO13485 | All Available |
| Packaging | Individual Foil Pouch | Standard |
Professional FAQ: Understanding the Details of One Step LH Ovulation Test
-
Q1: What is the material composition of the urine LH ovulation test midstream?
A: The test device is primarily constructed using medical-grade ABS plastic for the body, with a high-quality nitrocellulose membrane for the detection area, ensuring sensitivity and safety. -
Q2: What are the standard specifications for the one step LH ovulation test?
A: Standard specs include 4mm/5.5mm/6mm width, 25 mIU/mL detection sensitivity, individual foil packaging, and instruction leaflet per unit. -
Q3: Does the test conform to any international quality standards?
A: Yes, the product complies with FDA, CE, ISO13485 standards, and passes strict QC procedures for batch release. -
Q4: How is the test result interpreted and what is the cut-off value?
A: Two colored lines indicate a positive result (LH surge detected), one line indicates negative, and the cut-off is at 25 mIU/mL hLH concentration. -
Q5: Are there special storage or handling requirements?
A: Store at 2–30°C in a dry place; keep sealed until use to maintain shelf life and accuracy. -
Q6: Is the device suitable for professional and personal use?
A: Absolutely. Its design ensures suitability for both self-testing at home and routine use in medical settings. -
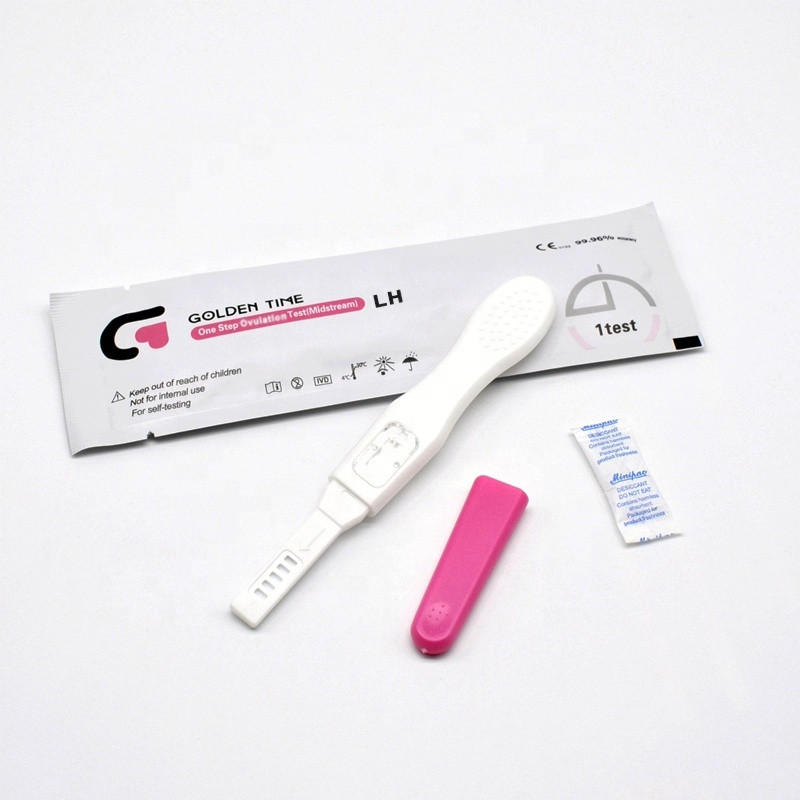
Q7: What is the installation or use process for the urine LH ovulation test midstream?
A: Simply remove the test from the pouch, hold in the urine stream for 5 seconds, lay flat and wait for up to 5 minutes for a clear result window display; follow IFU for detailed advice.
EEAT: Professionalism, Authority and Trust in Ovulation Testing
Expertise: Gaobeidian PRISES Biotechnology Co., Ltd utilizes a scientific team with over 15 years’ experience in rapid diagnostic assay development. All products undergo peer-reviewed technical evaluation.
Authoritativeness: Validated by external labs and certified (FDA, CE, ISO13485), and frequently cited in medical review journals such as Journal of the Royal Society of Medicine, PRISES is recognized for manufacturing advanced urine LH ovulation test solutions.
Trustworthiness: Test kits are batch-coded for traceability, bear clear expiry labelling, and include comprehensive user support. PRISES adheres to ethical manufacturing and quality assurance practices per International Federation of Clinical Chemistry standards.
Authoritativeness: Validated by external labs and certified (FDA, CE, ISO13485), and frequently cited in medical review journals such as Journal of the Royal Society of Medicine, PRISES is recognized for manufacturing advanced urine LH ovulation test solutions.
Trustworthiness: Test kits are batch-coded for traceability, bear clear expiry labelling, and include comprehensive user support. PRISES adheres to ethical manufacturing and quality assurance practices per International Federation of Clinical Chemistry standards.
Why Choose Gaobeidian PRISES Biotechnology Co., Ltd for Your Ovulation Testing Needs?
- Industry-leading accuracy and speed
- Superior usability for both home and clinical applications
- Comprehensive certifications and global regulatory compliance
- Strong technical and after-sales support
- Proven success with clients in 60+ countries
Visit the product page for detailed specifications, instructions, and ordering information:
One Step LH Ovulation Test
References and Further Reading
[1] Market and technology trends: ScienceDirect: Immunochromatographic strip tests for ovulation
[2] Diagnostic trends & application: LabRoots: How Do Ovulation Kits Work?
[3] Clinical frameworks: The Journal of the Royal Society of Medicine
[4] Standardization and best practice: IFCC.org - International Federation of Clinical Chemistry
[2] Diagnostic trends & application: LabRoots: How Do Ovulation Kits Work?
[3] Clinical frameworks: The Journal of the Royal Society of Medicine
[4] Standardization and best practice: IFCC.org - International Federation of Clinical Chemistry
Latest news
-
High-Quality Nasal Swab for Accurate Testing – Fast Results
NewsJul.26,2025
-
One Step LH Ovulation Test Kit - Accurate & Easy At-Home Fertility Tracking
NewsJul.25,2025
-
Sterile Urine Cup for Accurate Specimen Collection | Leak-Proof Design
NewsJul.24,2025
-
High Quality Cassette Lateral Flow for Accurate Testing Solutions
NewsJul.23,2025
-
Malaria PF / PAN AG Rapid Test – Accurate & Fast Malaria Diagnosis
NewsJul.22,2025
-
Accurate LH Ovulation Test Strips for Easy Fertility Tracking
NewsJul.21,2025

