
- Afrikaans
- Albanian
- Amharic
- Arabic
- Armenian
- Azerbaijani
- Basque
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Catalan
- Cebuano
- China
- China (Taiwan)
- Corsican
- Croatian
- Czech
- Danish
- Dutch
- English
- Esperanto
- Estonian
- Finnish
- French
- Frisian
- Galician
- Georgian
- German
- Greek
- Gujarati
- Haitian Creole
- hausa
- hawaiian
- Hebrew
- Hindi
- Miao
- Hungarian
- Icelandic
- igbo
- Indonesian
- irish
- Italian
- Japanese
- Javanese
- Kannada
- kazakh
- Khmer
- Rwandese
- Korean
- Kurdish
- Kyrgyz
- Lao
- Latin
- Latvian
- Lithuanian
- Luxembourgish
- Macedonian
- Malgashi
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Myanmar
- Nepali
- Norwegian
- Norwegian
- Occitan
- Pashto
- Persian
- Polish
- Portuguese
- Punjabi
- Romanian
- Russian
- Samoan
- Scottish Gaelic
- Serbian
- Sesotho
- Shona
- Sindhi
- Sinhala
- Slovak
- Slovenian
- Somali
- Spanish
- Sundanese
- Swahili
- Swedish
- Tagalog
- Tajik
- Tamil
- Tatar
- Telugu
- Thai
- Turkish
- Turkmen
- Ukrainian
- Urdu
- Uighur
- Uzbek
- Vietnamese
- Welsh
- Bantu
- Yiddish
- Yoruba
- Zulu
PRISES Biotechnology estas fabrikisto bazita en R&D, okupiĝanta pri evoluo, fabrikado kaj komerco de en vitro Diagnostikaj Reakciiloj (IVD) kaj Medicina Ekipaĵo, kiuj aprobis por fabrikado kaj komercado de IVD-produktoj de NMPA (CFDA) kaj funkciigita sub kvalito-sistemo de ISO 13485, plej multaj. de produktoj estis atestitaj kun CE-marko.
majoro
produktoj
-

Uncut sheet
Uncut sheet
Netranĉita folio por rapida provo
-
Plastic Cassette
Plastic Cassette
Plasta Kasedo Por Rapida Testo
-

Testo pri Infektaj Malsanoj
Testo pri Infektaj Malsanoj
Detects STDs, Hepatitis, H. pylori, Malaria, Dengue, Typhoid, Influenza and other diseases.
-

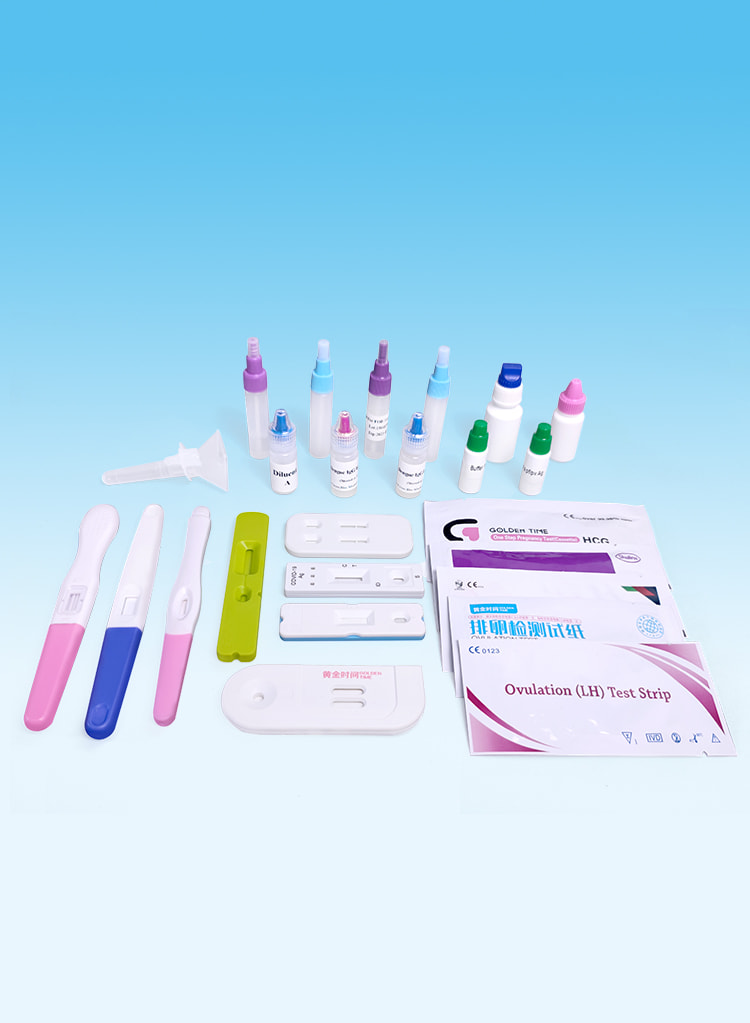
Testoj de Fekundeco
Testoj de Fekundeco
Effective detection of pregnancy and ovulation.
pri
PRISES
Our factory is founded in 2012 and located in Gaobeidian City, which is near Xiongan New Area and Beijing. It covers an area of 3,000 square meters, including class 1000,000 clean workshop with 1000 square meters, class 10 thousands of microbiological testing room with 500 square meters, well-equipped quality inspection rooms, research and development laboratories, etc.
-

10+
Business growth
10+
Business growthEstablished 11 years ago, the company has become a well-established and dynamic business through continuous efforts and improvements.
Rigardu Detalojn -

100000
Clean workshop
100000
Clean workshopClass 100,000 clean workshop 1000 square metres, Class 10,000 microbiological test room 500 square metres, fully equipped quality control room, R&D laboratory, laboratory, etc.
Rigardu Detalojn -

30+
Research staff
30+
Research staffThe company has a research and development team of 35 people who drive the technology and support the development of new products for the company.
Rigardu Detalojn -

20+
Consultant
20+
ConsultantWe provide the highest level of service to our customers, whether it's answering questions, solving problems or providing valuable advice, we deal with it promptly.
Rigardu Detalojn -

100+
Professionals
100+
ProfessionalsThe company has a large team of professionals responsible for managing, coordinating and controlling the operations of the various functions.
Rigardu Detalojn
novaĵoj kaj informoj

Fast Syphilis Test: Rapid, Reliable Diagnostics for Global Health
Discover how fast syphilis tests revolutionize early detection and treatment worldwide. Explore specifications, vendor options, benefits, and global applications.

Serology Syphilis Test: Global Importance and Latest Diagnostic Advances
Discover the essentials of serology syphilis testing, its global health role, innovations, and key benefits. Reliable, rapid diagnosis for better medical outcomes.

Diagnose Syphilis Test – Essential Screening & Diagnostics Explained
Discover how diagnose syphilis tests work, their global importance, practical uses, and innovations helping curb syphilis transmission worldwide.






